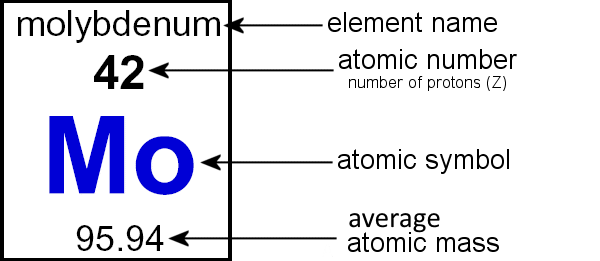
Atoms are made up of smaller particles, sub-atomic particles called protons, neutrons, and electrons. Each particle is defined by two properties: mass and electric charge. Mass just refers to how big or small the particle is. Charge comes in two vartieties: positive and negative. When two positive or negative charges meet, they feel a force pushing them apart. But when a negative and a positive charge are brought close together they feel a force pushing them together.
A proton (p+) is the most important part of an atom. The number of protons in an atom determines what kind of element it is. Protons have a mass of 1 atomic mass unit (amu) and a charge of +1. Protons are located in the atomic nucleus. Because they all have a positive charge there is a force pushing them apart. Thanks to another force, called the strong nuclear force, the protons are held together. They get a little help from neutrons. Elements are put in order of number of protons on the periodic table.
Neutrons (n0) are a necessary part of almost all atoms because they make atoms more stable by forcing the protons to stay together. They do this because they contribute to increasing the influence of the strong nuclear force. They have a mass of 1 amu and a charge of 0. That is, they are neutral. (Can you guess why they are called ‘neutrons’?) Atoms of an element can have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes.
Electrons (e-) balance out the electric charge of atoms because they have a charge of –1. Their mass is so small that it usually just is not counted. Electrons are located in a sort of cloud that extends away from the nucleus.
The mass of an atom is the sum of the masses of its parts. The charge on an atom is the sum of the charges of its parts.
| Subatomic Particles | |||
| Mass | Charge | Symbol | |
| proton | 1 amu | +1 | p+ |
| neutron | 1 amu | 0 | n0 |
| electron | 5 ×10-4 amu | –1 | e– |

| Electric Forces | ||
| + <—> + | ||
| + >–< – | ||
| – <—> – | ||
Questions
- Which subatomic particle weighs the least?
- Where are protons and neutrons located inside an atom?
- What is the name of the force that keeps the atomic nucleus from flying apart?
- What two subatomic particles are most important for finding the mass of a whole atom?
- What two subatomic particles are most important for finding the electric charge of a whole atom?
- An atom has 6 p+ and 7 n0. What is its mass in amu? What element is it?
- An atom has 7 p+, 7 n0. What is its mass in amu? What element is it?
- An atom has 11 p+, 12 n0. What is its mass in amu? What element is it?