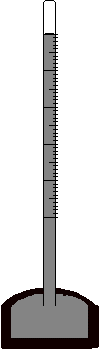
- The figure at right shows a mercury barometer. It is set up by filling a glass tube, closed at one end, with mercury. The tube is then submerged, open end down, in a pool of mercury. Which of the following statements is the best explanation of how this barometer works?
- Air pressure outisde the tube causes the mercury to move in the tube until the air pressure inside the and outside the tube is equal.
- Air pressure inside the tube causes the mercury to move in the tube until the air pressure inside and outside the tube is equal.
- Air pressure outside the tube counterbalances the weight of the mercury in the tube.
- The vacuum that is formed at the top of the tube holds up the mercury.
- If a barometer like the one above were built with water (D = 1.0 g/cm3) as the working fluid instead of mercury (D = 13.6 g/cm3), would the column of water be higher than, lower than, or the same as the column of mercury at 1.00 atm? If the level is different calculate the factor difference in the heights. Explain.
- For each of the three basic gas laws (Boyle’s, Charles’s, and Avogadro’s) sketch a graph showing the relationship between the two variables. Label the axes clearly and identify each law as a direct or an inverse proportion. For a law which is an inverse proportion, sketch another graph with a transformed variable to indicate how to make it into a direct proportion.
- You have a balloon designed to be filled with no more than 2.5 L of a gas. You fill it with 2.0 L of He gas at 25°C and 1.0 atm. You then try to hand it to a friend but clumsily release it and it floats into the upper atmosphere. Assuming a constant temperature determine whether the balloon will burst if it reaches a point where the air pressure is just 500. mm Hg.
- A too-curious student accidentally swallows a drop of liquid oxygen, O2. Liquid oxygen has a density of 1.149 g/mL. What volume of gas will form in this poor unfortunate’s stomach at body temperature (37°C) and 1.0 atm? Assume the drop of liquid O2 had a volume of 0.50 mL.
- Two 200.0 L tanks are to be filled separately with the gases helium and hydrogen. What is the mass of each gas needed to fill each tank to a pressure of 135 atm at 24°C?
- An ideal gas is placed into a large piston at a volume of 5.0 × 10 2 mL and temperature of 30.°C. It has a pressure of 710. torr. The gas is compressed to a volume of 25 mL, and the temperature is raised to 820°C. What is the pressure of the gas as a result of these changes?
- Consider the following chemical equation:
2NO2(g) --> N2O4(g)
If 75.0 mL of NO2 gas is completely converted to N2O4 gas with no change in temperature or pressure, what volume will the N2O4 occupy? - Air bags work by the explosive decomposition of sodium azide to form nitrogen gas:
2NaN3(s) --> 2Na(s) + 3N2(g)
What mass of sodium azide must be reacted to fill an air bag to 70.0 L at STP?