model kit. You have also drawn some structures of organic molecules. It is essential to the study of organic chemistry that you can use drawings to conjure up a mental image of the three-dimensional shapes of molecules. And if not mental models, then models you can draw from different perspectives in order to build your understanding of the structure. In this activity we will use the fact that molecules with the same formula can have many different possible structures to develop this capacity.
On this page there is a list of several molecular formulas. Your task is to build and draw as many different isomers for each molecular formula as you can. There are some rules about how atoms can be connected that you should remember:
| How Atoms Connect | |
| Element | Number of Bonds |
| C | 4 |
| H | 1 |
| O | 2 |
| N | 3 |
| F Cl Br or I | 1 |
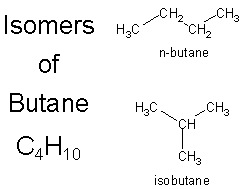
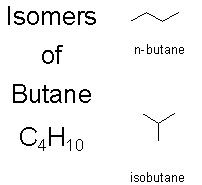
Above are the two possible isomers of butane.
The following chemical formulas have exactly the stated number of unique isomers. Build each one using your model kit and draw the result on the chart included with this handout.
- 2 isomers of C2H6O
- 3 isomers of C5H12
- 5 isomers of C6H14
- 4 isomers of C3H9N
- 4 isomers of C4H9Cl
Try just drawing isomers of the following two chemical formulas. If you can find all 9 unique isomers of heptane (C7H16) you can earn five percentage points on your next quiz. If you can find all 18 unique isomers of octane (C8H18) you can earn another 5 percentage points on your next quiz. This will not alter your ability to get five points for asking a question and for any possible bonus question.
- Build isomers of C7H16 (there are 9 possible isomers)
- Build isomers of C8H18 (there are 18 possible isomers)
To get credit for a model, fill in the table on the back of this page and the following pages with the formula, name (if known), and structure of each compound you build. There are enough spaces for all of the isomers listed above except for octane. If you wish to attempt to find the 18 isotomers of octane, you will need more paper.