|
molybdenum-96 Z = 42 & A = 96 Z = the number of protons Z = the atomic number A = the atomic mass A = the atomic mass number Z + n0 = A or A - Z = n0 |
|
|
molybdenum-96 Z = 42 & A = 96 Z = the number of protons Z = the atomic number A = the atomic mass A = the atomic mass number Z + n0 = A or A - Z = n0 |
|
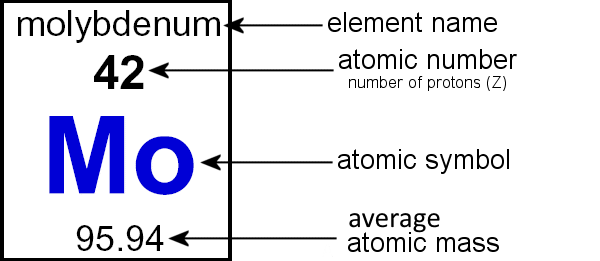
Fill in the table showing Z and A in the symbol, like in the example of molybdenum given above. Since these atoms are neutral they have the same number of electrons as protons. So the overall charge for all of them is 0.
For these problems you need to know how to read the names of
isotopes.
In aluminum-27 the word gives you the name of
the element. The number gives you the mass of the isotope. Find
the element on the periodic table. The atomic number (see key
above) tells you how many protons that element has. The mass
tells you the sum of protons plus neutrons for that isotope.
| Name | Symbol | Z No. of p+ |
A - Z No. of n0 |
A Mass |
No. of e– | Overall Charge |
| nitrogen-15 | 157N | 7 | 8 | 15 | 7 | 0 |
| oxygen-16 | 0 | |||||
| oxygen-18 | 0 | |||||
| magnesium-26 | 0 | |||||
| magnesium-24 | 0 | |||||
| phosphorous-31 | 0 | |||||
| calcium-40 | 0 | |||||
| calcium-42 | 0 | |||||
| silicon-28 | 0 | |||||
| silicon-30 | 0 | |||||
| neon-22 | 0 |