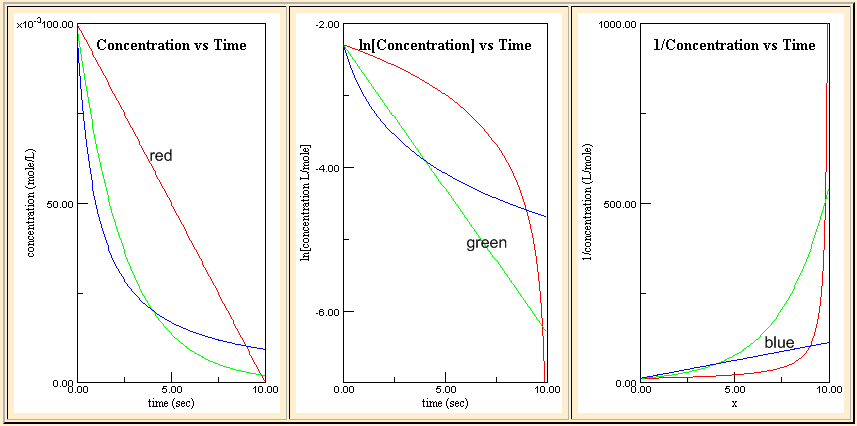
Kinetic Plot
Expression
Rate Constant
| - |
Δ[A] Δt |
= rate = k |
| t½ = |
[A]0 2k |
| - |
Δ[A] Δt |
= rate = k [A] |
| t½ = |
ln(2) k |
| - |
Δ[A] Δt |
= rate = k [A]2 |
| [A] = |
[A]0 1 + k t [A]0 |
|
1 [A] |
= k t + |
1 [A]0 |
| t½ = |
1 k[A]0 |