Electron
Domains
Pair
Domains
Electron
Domains
Bond
Angle(s)
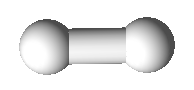
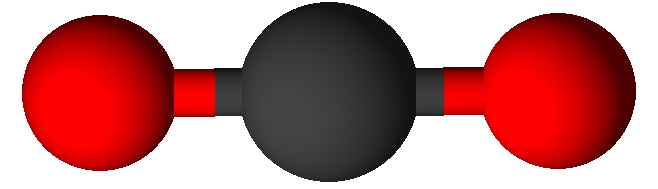
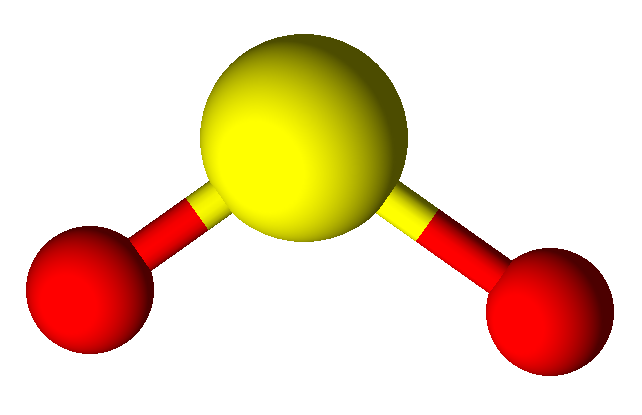
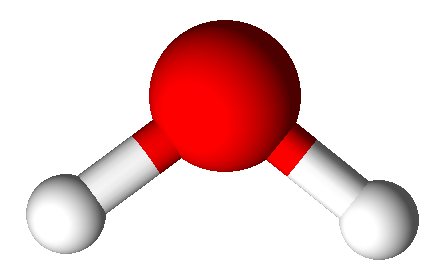
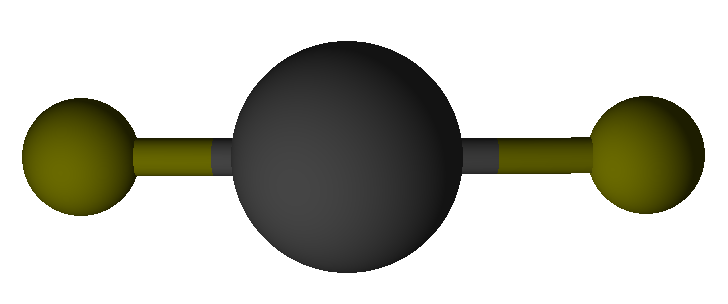
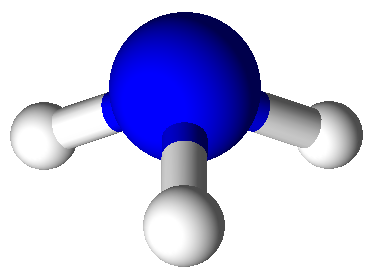
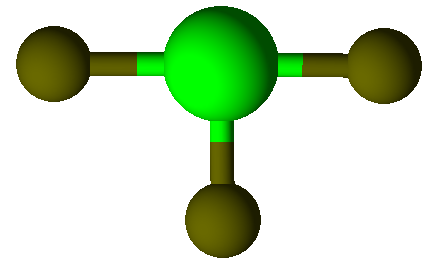
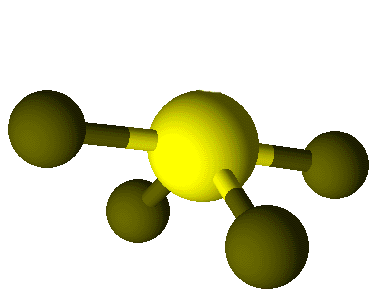
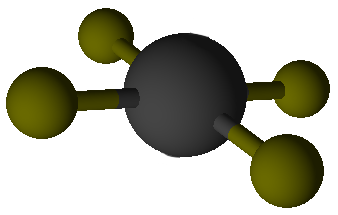
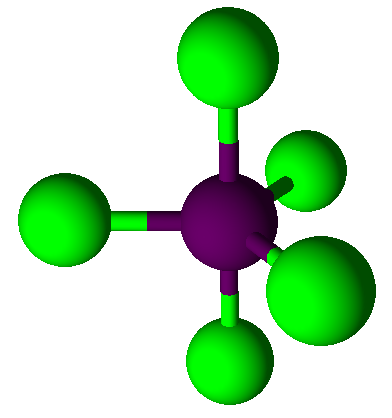
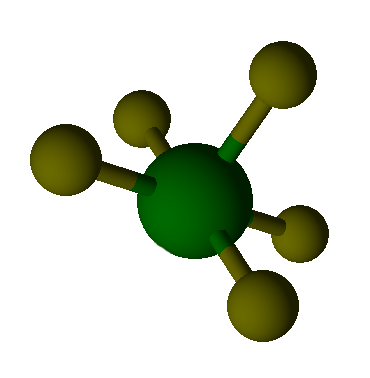
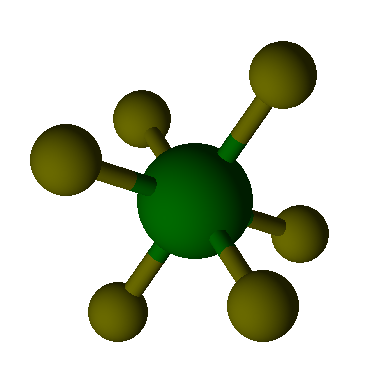
Valence Shell Electron Pair Repulsion model shapes, listed by number of bonds. Rows are colored according to the total number of electron domains.
| Number of Bonds on Central Atom | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|
Number of Lone Pairs on Central Atom |
0 | Linear | Linear |
Trigonal Planar |
Tetrahedral |
Trigonal Bipyramidal |
Octahedral |
| 1 | Linear | Bent | Trigonal Pyramidal | See-saw | Square Pyramidal | ||
| 2 | Linear | Bent | T-Shaped | Square Planar | |||
| 3 | Linear | Linear | |||||