 |
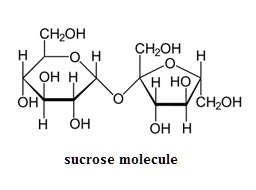
Table sugar is a carbohydrate known by the scientific name, sucrose. Its formula is C12H22O11. It is a disaccharide made of linked molecules of glucose and fructose. A disaccharide is a carbohydrate with two linked sugar molecules. Starches are composed of many linked sugar molecules and are known as polysaccharides.
Carbohydrates are so named because their empirical formulas may be written as a combination of carbon and whole number multiples of molecules of water. Sucrose for
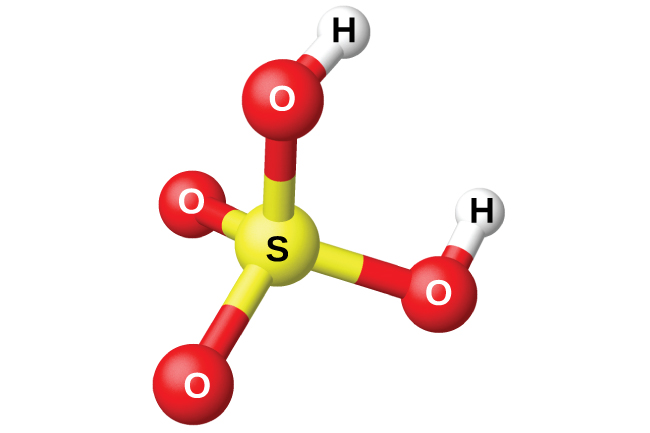 |
| Sulfuric Acid |
Concentrated sulfuric acid is 98% pure H2SO4 by mass. Its concentration may be expressed as 18 mol/L. Sulfuric acid is the chemical manufactured in the largest amount worldwide. Annual production is likely near 160 million tons. It is mainly used to dissolve ores in mining and phosphate rock in the manufacture of fertilizers. Because it is a diprotic acid (meaning it has two hydrogen ions, or protons, per molecule) sulfuric acid provides the equivalent of 36 mol/L for the concentration of H+. This is 45 times the H+ concentration of 5% table vinegar. Besides being an acid, H2SO4 is also a powerful dehydrating agent due to its highly exothermic enthalpy of solution.
The heat of reaction in this demonstration may be calculated by a Hess’s Law combination of reactions:
|
Combustion of Sucrose |
C12H22O11 + 12O2 → 12CO2 + 11H2O | ΔH = –5641 kJ |
|
Reversed Combustion of Pure C |
12(CO2 → C + O2) | 12(ΔH = +393.5 kJ) |
| Linear Combination |
C12H22O11 → 12C + 11H2O | ΔHrxn = –919 kJ |
In the demonstration as shown in class the use of 40 g of sugar results in the release of –104 kJ of heat. The heat of dilution for 98% sulfuric acid is –41 kJ/mol. If 40 mL are used in the demonstration then the amount of heat released will be –30 kJ. The water that evolves during the dehydration is turned to steam by this heat (the amount of water produced is 1.3 mol and requires 52.3 kJ to vaporize) and this is what inflates what is known as the ‘carbon soufflé’.
In case you missed the classroom demonstration there is a video I made which you can watch: https://youtu.be/xV3d94FrjzE. This demonstration is based on demonstration 1.32 on pg. 77 of Chemical Demonstrations, Vol. 1 by Bassam Z. Shakhashiri.