Whether an object floats in water or not depends on the object’s density compared to the density of the water. Water has a density of about 1 g/mL. If an object has a density less than 1 g/mL, such as a cork, the object will float. If an object has a density greater than 1 g/mL, such as a coin, the object will sink. Some objects can change whether they float or not by changing the mass without changing the volume. For example, if you overload a boat with cargo you may increase the density of the boat so much that it sinks. An increase of mass without an increase in volume increases density and when the density gets larger than 1 g/mL, then the object will sink. Off-loading cargo and reducing the mass inside the boat will decrease density again to the point where it is less than 1 g/mL and the boat can float.
Another way that density can affect flotation has to do with changes in volume for a constant mass. A piece of solid iron has a density of 7.9 g/mL or almost 8 times the density of water. And yet, all modern cargo and navy ships are made of steel, an alloy of iron! This is possible because the volume of the iron is increased by making hollow spaces inside it. By increasing the volume enough (by a factor of about 8) the iron ship can have a density less than 1 g/mL and it can float.
Temperature can also play a subtle part in deciding whether something floats or not. This is because temperature can affect the density of the water, making the water slightly more or less dense. For example, if you increase the temperature of the water, the density of the water decreases. For certain objects with a density very close to the density of water, this can have consequences for whether or not a floating object will continue to float or not. To understand why, we need to have a deeper understanding of the idea of temperature.
In a scientific sense, temperature is not how hot or cold something feels. Temperature is a measure of the average speed of the molecules of a material. Simply put, the higher the temperature, the faster the molecules of a material are moving. The speed of molecules determines how often they bump into one another. At low speeds they collide less often and at high speeds they collide more often. So as temperature increases, the number of collisions between molecules increases. The speed also determines how hard the molecules hit one another when they collide. The faster they are moving, the more force there is in each collision. So at low temperatures, molecules collide a bit less often and with a bit less force. And at higher temperatures, molecules collide more often and more forcefully.
An increase in temperature causes molecules to move faster. In the solid phase this means that they vibrate in place at a higher tempo. When molecules are in the liquid phase, they are clumped together but each molecule follows its own independent random path through the others. As temperature rises in the liquid phase it means that the molecules bump into each more often and with more force, causing them to be pushed
 |
 |
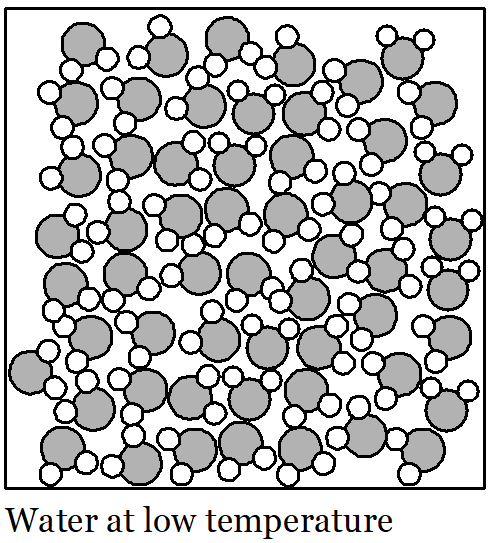
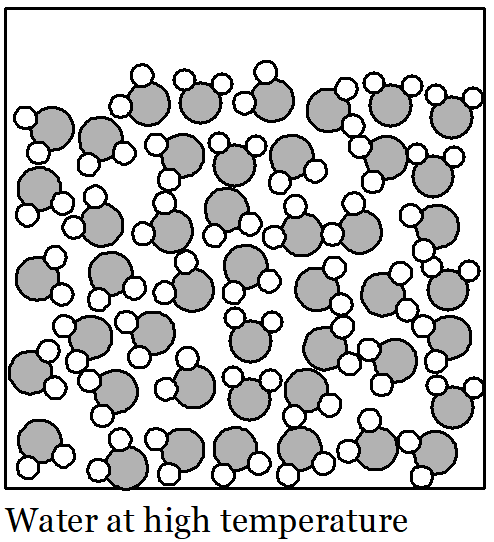
We are trying to understand the effect of temperature on the density of water. When water warms up, its volume expands. Since it has the same mass when its volume expands, its density decreases. What are the water molecules doing that explains this?
Picture a huge crowd of people in your mind. They are all standing and milling around and there isn’t much space between individuals. The people in the crowd are all walking slowly in random directions. If they keep moving slowly then they can stay quite close together. But if everyone starts walking a bit faster then they are going to bump into each other more often. If that keeps happening then all the people are going to push each other farther away so they have enough space to move. The floor-space covered by the crowd gets larger, even though there is the same number of people. This is a rough description of how water molecules behave in the liquid state. They all move about randomly and at low temperatures they move relatively slowly. If the water is heated up then the water molecules begin to move faster and push each other farther apart. As a result, the volume of the liquid increases, even though there are the same number of water molecules. So, the reason the water expands when its temperature rises is that the faster motion of the molecules pushes them farther apart, increasing the amount of empty space between the molecules. As temperature rises, volume increases. When the temperature of water falls its volume decreases because the molecules move more slowly and are not pushed as far apart by their motion. As temperature falls, volume decreases.
Density depends both on mass and on volume. If you add more mass, say by adding more water, then you also increase the volume and the density is unchanged. But it is possible to change the volume without changing the mass. Volume increases with increasing temperature. If you increase the volume without increasing the mass then this will have an effect on the density. The density will be lower if the volume increases. This is because there is an inverse proportion between the volume of a sample and its density. The bigger the volume, the smaller the density. Consider the equation for density
m
D = -----
V
at left.
As the value of V grows, the value of D must fall. And when the density of a liquid is lower, then the density of objects that float will also be lower. Or, if the objects do not change their density, then they will sink if their density is higher than the liquid’s density.
Answer the first five questions with your group. Answer the final question on your own. Then show your written answer to your teacher to obtain permission to test your explanation.