
|
|
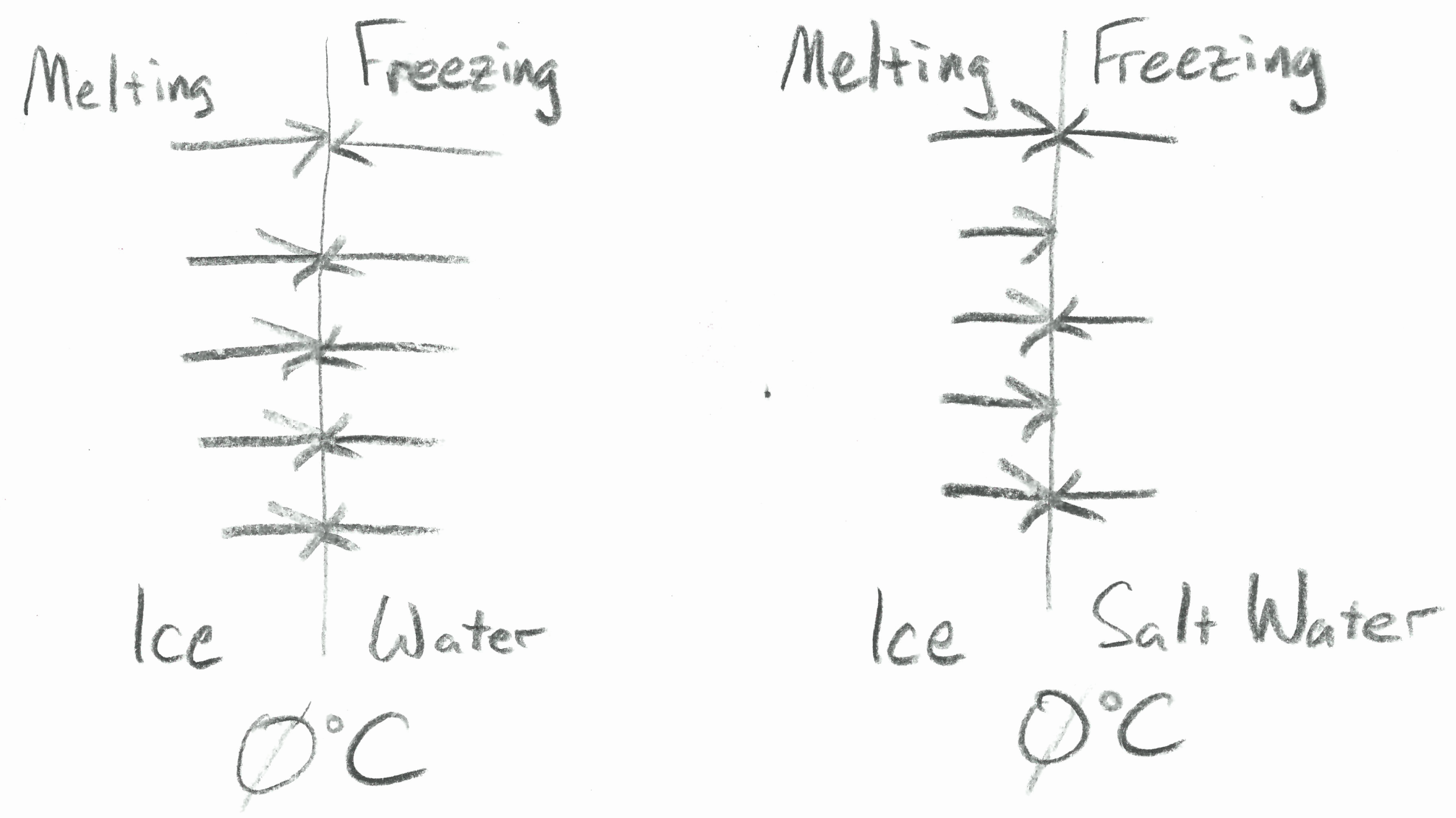
| There are equal freezing and melting rates for water at 0°C. | The rate of freezing is slower in salt water while the melting rate stays the same at 0°C. |

|
|
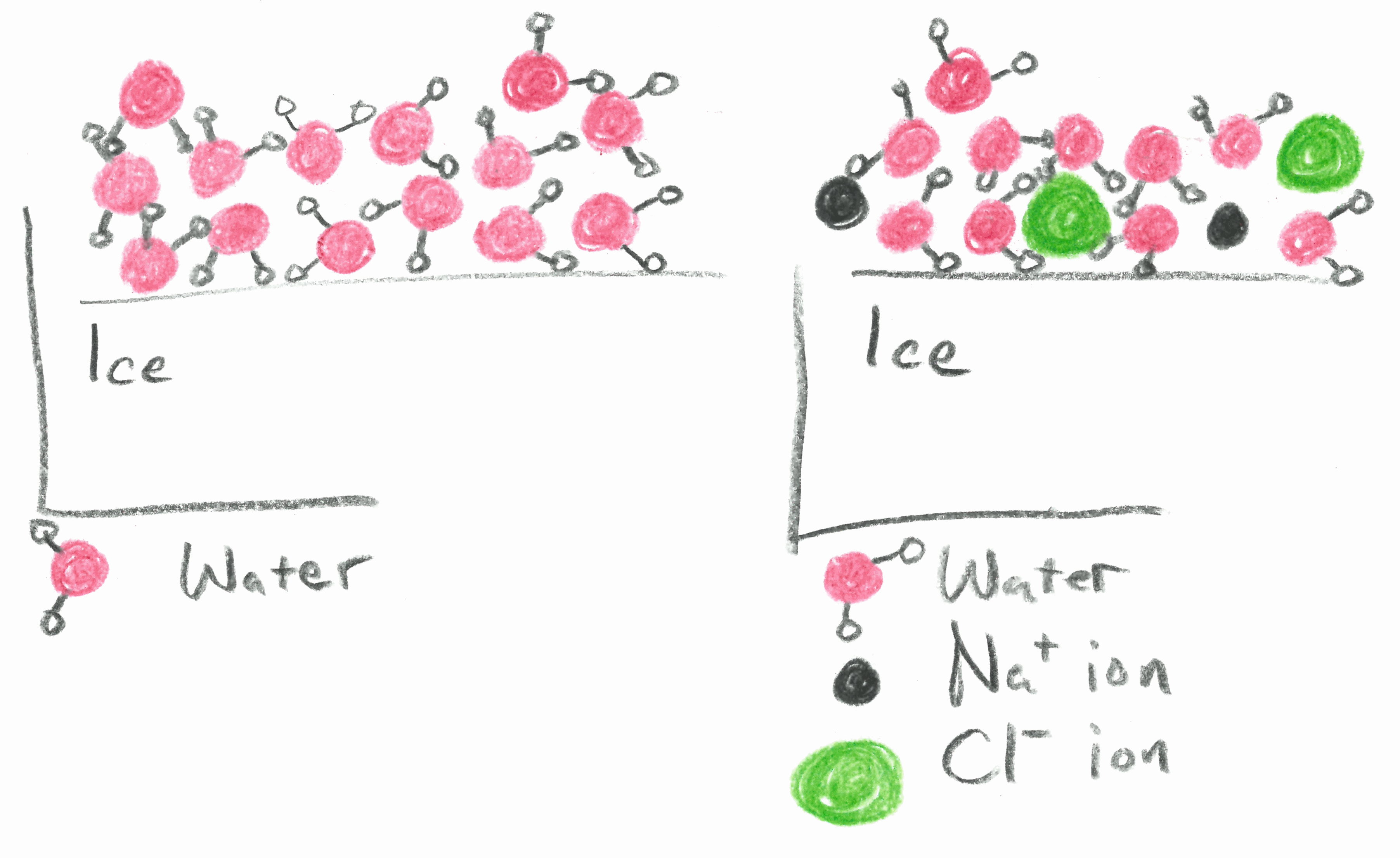
| Fourteen molecules of water in the liquid phase above an ice surface. | Only 10 molecules of water and 4 ions from NaCl. |
Water has a normal melting point of 0°C. This means that at that temperature, with no heat gained or lost from the surroundings, there is an equilibrium between melting and freezing. The rate at which molecules of water break their bonds to join the liquid is equal to the rate at which molecules form new bonds to join the solid. Breaking bonds consumes energy and if it were the only thing going on this would cause the temperature to drop. But new bonds are also forming and this is a process that releases energy. As a result, since bonds are made as often as they are broken, the temperature remains a constant 0°C. A schematic cartoon of these equal rates, and how they change in salt water, is shown in the upper image at right.
Things change when a solute is added to the water. If there is salt dissolved in the water it reduces the number of water molecules as compared with pure water. Imagine a very small volume of water in contact with ice that has only 14 molecules. Any of those molecules could stick to the surface, releasing heat as it forms the new bond. In salt water, of the 14 particles in our tiny volume perhaps only 10 are water molecules and the other 4 are ions of NaCl (2 Na+ and 2 Cl–). See the image below at right. The ions are not able to become part of the ice by forming bonds. As a result, the rate of freezing slows down even though the rate of melting remains the same. This is true if the temperature remains at 0°C. Though, as we will see, the temperature does not remain the same.
Because the freezing rate slows down, the rate at which energy is released also slows down. With fewer bonds forming, less heat is released. Meanwhile, the melting rate continues as before and consumes just as much energy as when there was no salt present. As a result, the temperature drops because more energy is being used up breaking bonds than is being given out by making bonds. To summarize: salt water has a lower freezing point because in salt water the number of water molecules has been reduced so that at the normal melting point more molecules melt than freeze. Salt causes the temperature of ice to drop because it changes the freezing rate so that more energy is consumed breaking bonds than is released by making bonds.
In the demonstration you will observe two things. First, how the temperature of an ice/water mixture changes when salt is added. Second, how a string and some salt may be used to pick up an ice cube.
This demonstration is based on demonstration 9.21 “Getting Colder: Freezing Point Depression” pg. 290 of Chemical Demonstrations, Vol. 3 by Bassam Z. Shakhashiri.