Demo Notes:
Synthesis and Explosive Properties of Nitrogen Triiodide (NI3)
Note: I will not include here instructions on how to carry out the demonstration. My source was the incomparable Chemical Demonstrations, Vol. 1 by Bassam Z. Shakhashiri. Specifically, demonstration 1.39 on page 96. I used the discussion in that book and this page at Chemistry World Magazine, as well as the Wikipedia article, as sources for my remarks.
Nitrogen triiodide is a high explosive that is so sensitive that the slightest touch will set it off. It has no practical uses because it cannot be stored or transported. Impractically, it is usually made for classroom demonstrations which are very entertaining.
But first, a bit about the chemistry. Nitrogen triiodide (chemical formula: NI3) is made using concentrated ammonium hydroxide solution and solid iodine crystals. The ammonium hydroxide solution is a 14.8 M solution of ammonia. Ammonia (chemical formula: NH3) is a gas as a pure substance but has an extremely high solubility in water. It reacts with the water to form ammonium ions (chemical formula: NH4+) and hydroxide ions (chemical formula: OH–). The viscous liquid gives off an overwhelming odor of ammonia and is highly corrosive due to its very high alkalinity. The other ingredient in synthesizing nitrogen triiodide is crystalline iodine (chemical formula: I2). It is a pure element from the halogen family of elements, group 17 in the periodic table. It is the only halogen to be a solid at room temperature. At slightly above room temperature the iodine sublimes readily, producing a dark purple vapor. Like all the halogens, iodine is a strong oxidizer and exposure to the solid or the vapors can cause burns. The vapor is particularly harmful to the respiratory system.
When you mix the ammonium hydroxide solution with iodine crystals they react to form a dark brown solid, which is the explosive material. While wet it can be safely handled. It is only once it has had a chance to dry that it becomes unstable and touch-sensitive.
The chemistry of the formation of that dark brown solid is actually quite complicated. A direct synthesis of nitrogen triiodide which produces the pure substance can apparently be performed using boron nitride and iodine fluoride. In this experiment, however, a variety of intermediates and side reactions take place. Here is a look at the expected reactions:
NH3 + H2O + I2 ⇔ NH4I + HOINH3 + 2HOI ⇔ NHI2 + 2H2O
NH3 + 3HOI ⇔ NI3 + 3H2O
2NH3 + 3HOI ⇔ NH3·NI3 + 3H2O
It is the adduct of ammonia and nitrogen triiodide (chemical formula: NH3·NI3) which is understood to be involved in the explosion. The two molecules associate with each other with a bond that is weaker than a covalent bond. The solid structure of this adduct is said to consist of chains of this form: -NI2-I-NI2-I-NI2-I-… surrounded by ammonia molecules. When kept in the dark and damp with ammonia this product is stable. Once it dries it is explosively unstable.
The dry nitrogen triiodide decomposes to form nitrogen gas, iodine vapor, and ammonium iodide:
8NH3·NI3 ⇒ 5N2 + 6NH4I + 9I2In the presence of water vapor from the air the ammonium iodide also decomposes:
NH4I + H2O ⇒ I2 + NH4OHThe rapid production of so many moles of nitrogen gas provides the power for the explosion. The exact amount of energy released is difficult to measure, to say the least, and since some thermodynamic data are not available it is also not possible to calculate it.
Now, explosions can be very entertaining but the question remains: why is nitrogen triiodide so extremely unstable? The answer has to do with the difference in size between iodine atoms and nitrogen atoms. In order to form a bond to the nitrogen atom the three iodine atoms have to crowd very close together. This brings their outermost electrons into contact, causing a repulsive force.
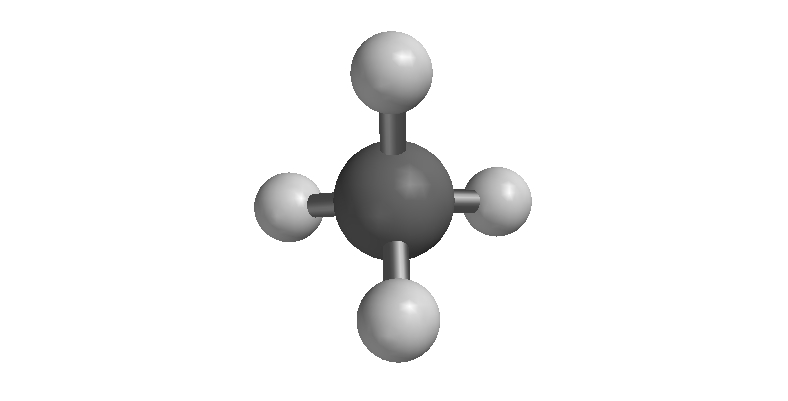 |
| Methane (CH4) |
As proof of this idea, consider the bond angles in the nitrogen triiodide molecule. The idealized angle between iodine atoms in this molecule is 109.5° because the three iodine atoms and the lone pair on the nitrogen atom require a tetrahedral electron domain geometry. Methane, for example, has perfect tetrhedral angles (see image at right). In a molecule like nitrogen trifluoride (NF3) the bond angles are 101.9°. This angle is smaller than the ideal angle of 109.5° because there is a non-bonding pair of electrons on the nitrogen atom which pushes the fluorine atoms together.
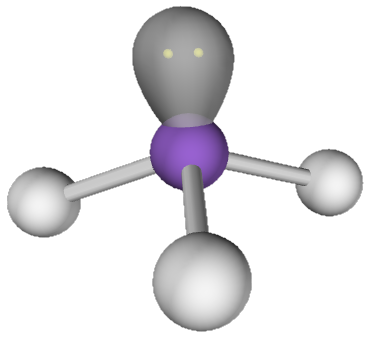
|
| Tetrahedral Geometry with Lone Pair |
By contrast, the bond angles in nitrogen triiodide are 115.8°. This is over 6° larger than the ideal angle of 109.5°. That this angle is larger than the ideal angle, despite the presence of the lone pair on nitrogen, goes to show that the iodine atoms are crowded much too close together. They repel one another and weaken the bonds between the nitrogen atom and the iodine atoms. Because these bonds have been weakened the substance is highly unstable. This sort of situation is called steric strain. Steric strain refers to the instability that occurs in molecules when one or more atoms or groups of atoms is forced to occupy the same volume.
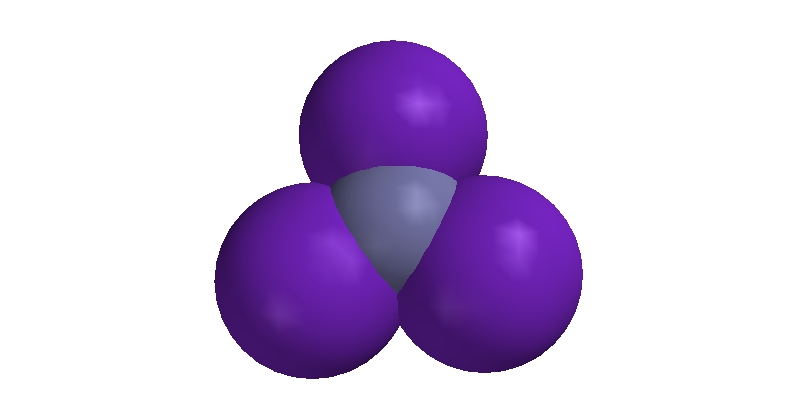
|
| Space-filling Model of Nitrogen Triiodide |
Here are a few other things I feel I should mention:
-
Safety
- As always, no one but a trained chemist should perform this demonstration.
- Aqueous ammonia, also known as ammonium hydroxide solution, is toxic, foul-smelling, and highly alkaline and therefore corrosive. The vapors produced by this concentrated solution can be overwhelming so it should be used in a fume hood or with adequate ventilation. Contact with skin and eyes should be avoided as it will dissolve skin and destroy eye surfaces.
- The solid iodine can cause burns because it is highly oxidizing. The vapor is harmful to the eyes and respiratory system.
- The sounds produced by the explosion of nitrogen triiodide can be extremely loud. Hearing protection and fair warning to spectators is advised.
- Ensure adequate ventilation to avoid breathing the iodine vapors produced in the explosions.
-
Cleanup
- Rinse out the beaker in which the synthesis was carried out with plenty of water and wash down the drain.
- Throw used filter papers in the trash.