 |
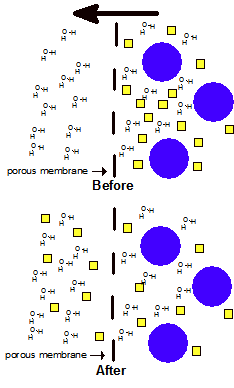
| Top: a bag containing a solution of two kinds of molecules is placed into water. The bag has pores only big enough to allow the small molecules to pass. Bottom: the small molecules have equal concentrations on both sides. |
Looking at a model of a process can help you to understand the process. Kidneys use the process of diffusion across a membrane to filter out unwanted products of metabolism and excess water. Separation of molecules using membranes is called dialysis and dialysis is something you can do in the lab. The idea in this lab is to explore dialysis to get a basic idea of how it works and then to use that knowledge to do some further research.
You have already become somewhat familiar with dialysis from reading an article in ChemMatters (4/2001). In this lab you will perform a few demonstrations of the key concept important in dialysis: diffusion. To make it real science you will also do some research into the following questions:
 |
| Top: a bag containing a solution of two kinds of molecules is placed into water. The bag has pores only big enough to allow the small molecules to pass. Bottom: the small molecules have equal concentrations on both sides. |
Diffusion is a molecular-level process in which molecules pass through a porous membrane. The membrane has holes big enough to let some molecules pass through but small enough that others can’t pass. Molecules that can fit through will travel through the membrane in the direction that takes them from higher concentration to lower concentration. When the concentration of the molecule is the same on both sides of a membrane then diffusion happens at the same rate in both directions. Water molecules generally can pass in both directions through a membrane but this is not always the case.
Diffusion can be used to separate molecules of different sizes. See the illustration at right. An analogy might be a screen door: dust and dirt can pass through but bugs are kept out.
A definition, in case you need it: The concentration of a chemical is just a measure of how much of it there is in a volume of liquid or gas. A practical example: an 8-oz. glass of water with one spoonful of sugar tastes a lot less sweet than the same glass of water with 5 spoonfuls of sugar.
Cornstarch belongs to a group of biological molecules called polysaccharides. Starches in general are large molecules made up of tens to hundreds or even thousands of individual sugar molecules. These large molecules usually have twisted and convoluted structures with a lot of pockets in them. Tincture of iodine contains I2 molecules and is a light brown color. When you add tincture of iodine to a starch solution it forms a complex with the starch by sitting in the many pockets in the molecule. The complex has a characteristic dark blue color. Because this complex forms it is possible to use iodine to determine whether any starch is present.
The procedure for this lab is mostly up to you. After your teacher gives you an introduction to the lab it is your decision about how to proceed. Some things to keep in mind:
Here are a few things you need to know how to do in order to be successful in answering the objective questions.
ColorsBefore you start any dialysis it would be wise to take a look at what color the iodine solution is (see below for how to make it. Also, you should take a look at the range of colors produced when you add cornstarch to an iodine solution. Make yourself familiar with what happens when iodine and cornstarch meet.
This is your standard cornstarch solution. When trying to find out how concentration affects diffusion you should use a measured amount more or less than the 3 g of cornstarch used here.
This is your standard iondine solution. Place baggies containing cornstarch solution into this bath to start the dialysis process. It can be re-used for more than one trial so do not throw it out!
To do dialysis for any of your experiments pour some cornstarch solution into a baggie, seal the bag and then let the bag rest in the iodine solution. Be cautious and do not let any cornstarch solution get on the outside of the bag. When taking observations of the diffusion process let the bag stay in the iodine solution but take it out every two or three minutes to look at it. It may take as long as 15 minutes for the process to be complete.