Electrified Objects
Objective
There are several objectives in this lab activity. First is to investigate how objects may obtain electrical charges by rubbing different materials together. Second is to observe how charged objects interact with one another. Third is to observe how charged objects interact with uncharged objects.
Materials
dark plastic stripsclear plastic strips
scrap paper
 cloth
clothtape
ringstand with horizontal bar
Background
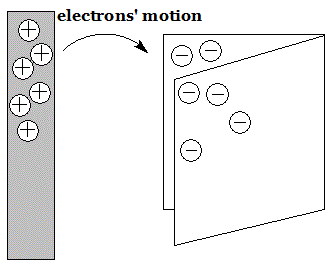
Since the eighteenth century there has been systematic study of electric charge. This was carried out by rubbing two different materials together, which causes those materials to obtain an electrical charge. In fact, this knowledge has been around for far longer. The ancient Greeks knew about this phenomenon. We get our modern word electron from the Greek word for amber because rubbing amber with cloth caused it to become charged up. Electricity-through-rubbing is called triboelectricity. When one material is rubbed with another the friction causes electrons on one to move to the other. The material that ends up with additional electrons obtains a negative charge. When electrons are removed from a material it ends up with a positive charge. In the illustration at right a piece of paper has removed some electrons from a piece of plastic, leaving both with a net electric charge equal in size but opposite in sign.
Electrons are negatively charged particles that can easily move from one atom to another. Atoms also contain positively charged protons and neutral neutrons. The protons and neutrons make up an atomic nucleus. Atomic nuclei cannot be moved from their positions in solid materials nearly as easily as electrons.
The electrons are attracted to atomic nuclei because opposite charges experience an attractive force. Electrons repel each other, however, because objects with the same kind of charge repel each other. Similarly, the positively charged atomic nuclei experience a repelling force away from each other. Matter is held together because the electrons are arranged around atomic nuclei in such a way that the attractive forces are stronger than the repulsive forces.
Materials may be classified as either electrical conductors or electrical insulators. Electrical conductors allow electrons to pass through them easily but insulators prevent the travel of electrons. When charges are separated by rubbing two different materials together the electrons that build up on the negatively charged object will all repel each other. If brought into contact with a conductor they will move into it to get as far from each other as possible. Charges that can move will always move in a such a way that they find an opposite charge. It is for this reason that matter is made up of equal numbers of positive and negative charges. Most of the time, material objects have a neutral charge.
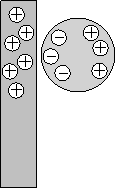
One thing that could lead to confusion in this lab is a phenomenon called charge induction. When a charged object is brought near an uncharged object it causes the electrons in the uncharged object to become polarized. That is, the electrons move toward the external charge if it is positive or away from it if it is negative. As a result, there is always an attraction between a charged object and an uncharged one. In the illustration at left a postively charged rectangle is brought near a neutrally charged ball. This causes electrons in the ball to move toward the positive charges and therefore the two objects attract each other. It is because of charge induction that you can rub a balloon on a sweater or your hair and stick it to the wall.
Safety
This lab has minimal safety considerations.
Procedure and Questions
Using a piece of tape, hang a piece of the dark plastic from a horizontal bar. Also hang a piece of clear plastic. Be sure that both pieces of plastic hang so that they can swing freely without twisting. Briskly rub both hanging strips with a piece of folded paper. Perform each of the described actions and answer the questions using complete sentences.
- Briskly rub a second piece of dark plastic with a piece of paper. Bring it slowly near the hanging dark piece of plastic (which you have also just rubbed with paper). What do you observe?
- Bring the rubbed piece of dark plastic near the hanging piece of clear plastic (which you have also just rubbed with paper). What do you observe?
- Briskly rub a second piece of clear plastic with a piece of paper. Bring it slowly near the rubbed hanging dark piece of plastic. What do you observe?
- Bring the rubbed piece of clear plastic near the rubbed hanging piece of clear plastic. What do you observe?
- Summarize your observations about how the rubbed pieces of plastic interact using words like attract and repel. Be sure to describe all possible interaction between the two types of plastic.
- What type of charge does each type of plastic have? Assign the names positive and negative to each type of plastic when rubbed with paper.
- Based on the background information and your in-class discussion prior to doing this activity, write a hypothesis about how electrons are moving between the paper and the plastic. Which type of plastic is gaining electrons and which type is losing electrons when rubbed with paper?
- If you switched which type of plastic you called positive and which you called negative would it have any effect on how they acted? Why or why not? Reverse your naming system and re-do the experiments. Is there any difference? Why?
- Tear up a small scrap of paper to make about 6 - 10 very small pieces. Charge up one of the loose pieces of plastic by rubbing it with paper. Hold the charged plastic near to the pile of small scraps of paper. What happens?
- Why are the small un-charged pieces of paper attracted to the charged plastic?
- Obtain a plastic ruler, comb, piece of glass, or a piece of styrofoam and rub two or more of them with paper. Based on their interactions with the charged-up hanging strips of plastic what charge do each of these objects have when rubbed with paper? By doing experiments, find out whether the objects still have the same charge when rubbed on your clothes or a piece of wool. Write the results of these experiments below.