Objective
The lab work has two objectives: first, to gain some experience with how radioactivity really works. Students will collect data about levels of activity, the effect of distance on radioactivity counts, and shielding. Second, students will learn about how a Geiger counter works (also called a Geiger-Müller counter).
Overview
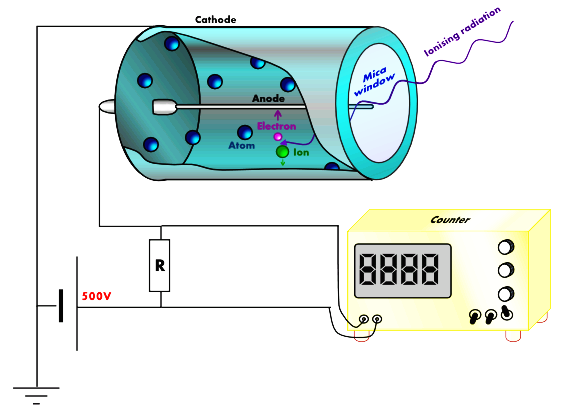
In this activity you will collect data as part of a demonstration of a Geiger counter. Geiger counters are designed to measure the number of nuclear decays per minute of radioactive substances. Usually the displayed numbers are in fact only proportional to the true number of radioactive decays per minute. Geiger counters are sensitive to ionizing radiation
Radiation from a given sample of a substance is characterized by three features: (1) how intense it is, (2) how strongly ionizing it is, and (3) how easily it can be blocked. In this lab you will collect data about each of these three features.
Background
Radioactivity that can be detected by Geiger counters comes in three types, which you have already learned about: Alpha radiation, beta radiation, and gamma radiation. Alpha and beta radiation involve the ejection of a particle with mass from the nucleus. Alpha particles are helium-4 (42He or 42α) nuclei moving at extremely fast speeds. Alpha particles are not atoms because they do not have electrons. Beta particles are electrons (0–1β– or 0–1e–) moving at immensely high speeds. Gamma particles are photons (00γ) emitted by a nucleus in an excited energy state. Gamma particles have no mass and because they are a form of electro-magnetic radiation (light) they are also called gamma waves.
All three types of radiation (α-rays, β-rays, and γ-rays) cause ionization. Ionization is an atomic-scale process in which atoms lose one or more electrons and therefore come to have a positive charge. When a radioactive particle strikes matter it cause electrons to become ionized. These electrons can then cause damage to neighboring atoms. Also, the positively charged atoms and molecules need to become neutral again and so steal electrons from neighboring atoms and molecules. This causes a cascade of electron-stealing as more molecules are affected by the lack of an electron. In living things these ionized molecules no longer work properly and can make cellular processes fail to function. Also, ionization can lead to damage to DNA, causing mutations and (with long exposure) cancer. Geiger counters take advantage of this ionization because the motion of electrons can be detected as an electrical current. Each ionization event (i.e., whenever a radioactive particle passes through the detector tube window) causes a spike in the current in the Geiger counter. This happens because when the ionizing radiation strikes an atom of the low-pressure gas inside the tube it causes that atoms to lose an electron. The high voltage inside the tube accelerates that electron toward the positively charged anode. This creates the electrical current detected by the circuitry in the counter. Geiger counters typically display the number of counts per minute. Each count represents the decay of one radioactive atom. Some counters display a number which the user must multiply by 10, 100, or 1,000 to get the true number of counts per minute.
The intensity of radiation from a sample depends on the half-life of the substance and the number of atoms that are present. In general, if the substance has a short half-life the intensity will be higher. If the half-life is long, the intensity will be lower. This is because half-life is a measure of the rate of decay. If an isotope has a short half-life it means that the sample decays to half of its original intensity more quickly than an isotope with a longer half-life. The number of atoms present also plays a role in how intense the radiation will be. If there are a large number of atoms then there are more atoms that can decay during a given period of time. If the sample is small then there will be correspondingly fewer decays per minute. If the sample is large, even for atoms with a very long half-life, the radiation may still be very intense. All isotopes of uranium have very long half-lives, some of them several billion years. Even so, a large enough piece of pure uranium still produces very intense radiation because there are so many atoms.
The intensity of radiation drops quickly with increasing distance. As the distance from the source increases the intensity decreases with the square of the distance. So if you double the distance the intensity decreases to 1/4 of the original amount. If you triple the distance the intensity decreases to 1/9; quadruple it and it decreases to 1/16…and so on. The best way to describe this relationship is to say that the intensity is inversely proportional to the square of the distance. Using mathematical symbols: I ∝ 1/d2 where I is intensity in counts per minute and d is distance.
The ionizing power of radiation depends on the type of radiation. Alpha particles are really the nuclei of helium-4 atoms. As such, they have a positive two charge. They are the most massive kind of radioactive decay particle with a mass of 4 amu. Given their large mass and high electrical charge they are by far the most ionizing kind of radiation. Beta particles are really electrons (or, in the case of beta-plus particles, they are positrons). Electrons and positrons have a mass almost 2,000 times smaller than the mass of a hydrogen atom. However, they have a full unit of electrical charge and this leads to beta radiation being the second-most strongly ionizing form of radiation. Gamma rays are photons of very-high-energy light. They have zero mass and zero charge. As a result, they are the least ionizing form of radiation. Other forms of electro-magnetic radiation (in other words, light) can also cause atoms to become ionized. Ultraviolet light and x-rays can also cause ionization. This is why exposure to sunlight, which includes UV light, causes sun burns. It is also why the number of x-rays a person receives each year should be limited. Other forms of light cannot cause ionization and are therefore extremely unlikely to cause harm. For example, the microwave radiation used to allow cell-phone communication has far too little energy per photon to cause the kind of genetic damage that could lead to cancer.
Shielding can reduce the intensity of radiation as well. Alpha-rays are the easiest to shield against and they can be blocked with a piece of paper. This means that their strong ionizing power is usually not important, at least for a sample outside the body. In fact, alpha particles can barely penetrate skin. But if an alpha-emitter should find its way inside the body it is by far the most damaging. Beta-rays are more penetrating and will penetrate up to 1 cm into a human body. They can be blocked with a relatively thin piece of metal such as a few layers of aluminum foil. Additional concerns with beta-rays involve the x-rays produced by the interaction of the particles with the shielding but we won’t study that in this lab. Gamma-rays are by far the most penetrating type of radiation. They are similar to x-rays but are more powerful. Gamma-rays can pass all the way through a human body and are difficult to shield against. The best shielding is done with atoms that have heavy nuclei, such as lead (average atomic mass = 207 amu). Lead is best for shielding against gamma-rays but anything will do, as long as it is thick enough.