In this lab students will play with the hydrogen simulator at the Nebraska Astronomy Applet Project (NAAP). The simulator is on the Hydrogen Energy Levels Module page (http://astro.unl.edu/naap/hydrogen/hydrogen.html). While doing so they will learn about the relationships between the wavelength, frequency and energy of photons of light. Also, they will explore how photons interact with the quantum energy levels of the electron in a hydrogen atom.
In this lab you will review a few facts about the nature of light. First, the wavelength and the frequency of light are related by an inverse proportion: c = λf. (Remember, c = 3.00 × 108 m/s when wavelength, λ, is in meters and frequency, f, is in 1/s, also called Hz). Second, the frequency of light and the energy of a single photon at that frequency are related by a direct proportion: E = hf. (Remember, E is the energy in joules, J, of one photon of light with frequency, f, in Hz; h is Planck’s constant: 6.626 × 10-34 J· s).
Also in this lab you will explore what the atomic processes of absorption and emission look like. You have been told, but probably do not yet fully believe, that atoms only absorb light in discrete amounts: quantum energies. In this activity you will be able to experience it yourself. In the process you will be able to confirm the visible-light spectrum of hydrogen gas, shown below:

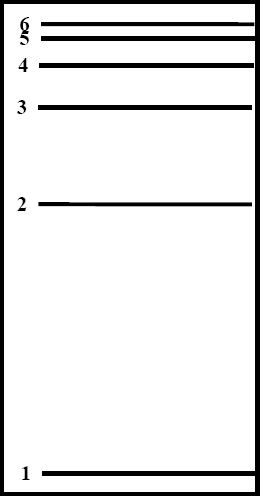
The electron in a hydrogen atom can orbit the nucleus (a single proton) at different distances called Energy Levels. The closer the electron is to the nucleus the lower its potential energy and the farther it is from the nucleus the more potential energy it has. When the electron is as close as quantum physics will allow it to get then it is said to be in the Ground State. The Ground State is called energy level 1. All other orbits around the nucleus are called Excited States. They are numbered up from level 1 in the energy level diagram shown at right.
When an electron moves from a lower energy level to a higher energy level it is called excitation. For this to occur the atom must absorb exactly the right amount of energy: the quantum energy difference between the two energy levels. In this simulation this is only possible when the atom absorbs a photon. Absorption is the process in which an electron in an atom jumps to a higher energy level by using up the energy of a photon. Electrons can be moved to higher levels in other ways (such as by heating up the atoms) but in this simulation absorption is the only possible way.
When an electron moves from a higher energy level to a lower energy level it is called de-excitation. When this happens the electron releases an amount of energy that exactly matches the difference in energies between the two energy levels by emitting a photon. The photon will always have exactly the quantum energy represented by the difference between the energy levels. Emission is the process in which an electron in an atom jumps to a lower energy level by emitting a photon.
Electrons can only occupy certain energy levels with well-defined quantum energies. There is a lowest energy level (the Ground State) and a highest energy level. Beyond the highest energy level an electron becomes free of the atom. When the energy of a photon exceeds a certain amount then no matter what energy the photon has it will cause the electron to fly away from the atom entirely. This event is called Ionization. Ionization occurs when an electron absorbs a photon with more energy than the energy that would take it to the highest available energy level. When an electron joins up with the atom again it is called Recombination.
A note about units. Wavelength must be measured in meters (m) and frequency must be measured in Hz (1/s) to use the c = λf formula. Energy calculated using E = hf is in joules (J). In this simulator wavelength is given in more convenient units: nanometers (nm) and micrometers (µm). One meter equals 1 × 109 nm and 1 × 106 µm (1 m = 1 × 109 nm; 1 m = 1 × 106 µm). Also, it is important to realize that 1 µm = 1,000 nm. Frequency is given in Hz in the simulator but the energy values are given in electron-volts (eV). One eV is equal to 1.602 × 10-19 J. The eV is a much more convenient unit when you are talking about individual atoms and that is why it is used here. Regardless of the unit, frequency is still directly proportional to energy.
| Range | Wavelength
(common units) |
Wavelength
(λ, m) |
Frequency
(f, Hz) |
Energy
(J) |
Energy
(eV) |
| Infrared (IR) | 0.750 - 1,000 µm | 7.5 × 10-7 - 1.0 × 10-3 m | 4 × 1014 - 3 × 1011 Hz | 2.65 × 10-19 - 2.00 × 10-22 J | 1.65 - 1.24 × 10-3 eV |
| Visible (Vis)
ROYGBIV |
400 - 700 nm | 4.0 × 10-7 - 7.0 × 10-7 m | 7.5 × 1014 - 4.3 × 1014 Hz | 5.00 × 10-19 - 2.84 × 10-19 J | 3.1 - 1.8 eV |
| Ultraviolet (UV) | 10 - 400 nm | 1.0 × 10-8 - 4.0 × 10-7 m | 7.5 × 1014 - 3 × 1016 Hz | 2.00 × 10-17 - 5.00 × 10-19 J | 124 - 3.1 eV |
In the visible part of the spectrum (from 400 nm - 700 nm) there are recognizable colors. Light with a wavelength of 400 nm is violet while light with a wavelength of 700 nm is red. You can remember the colors and order of the rainbow using the mnemonic ROYGBIV. You can think of it as a name: Roy G. Biv. The colors, of course, are Red, Orange, Yellow, Green, Blue, Indigo, Violet. They are listed in order of increasing frequency and decreasing wavelength: violet has the shortest visible wavelength and red has the longest.
The very longest wavelengths of IR light cross over into the microwave region of the spectrum. Microwaves have still lower energies and still smaller frequencies.
Light with a frequency higher than that of UV light have shorter and shorter wavelengths. The wavelengths of x-rays are just shorter than UV. Gamma rays have still shorter wavelengths. In order of increasing energy: UV, x-rays, gamma rays.
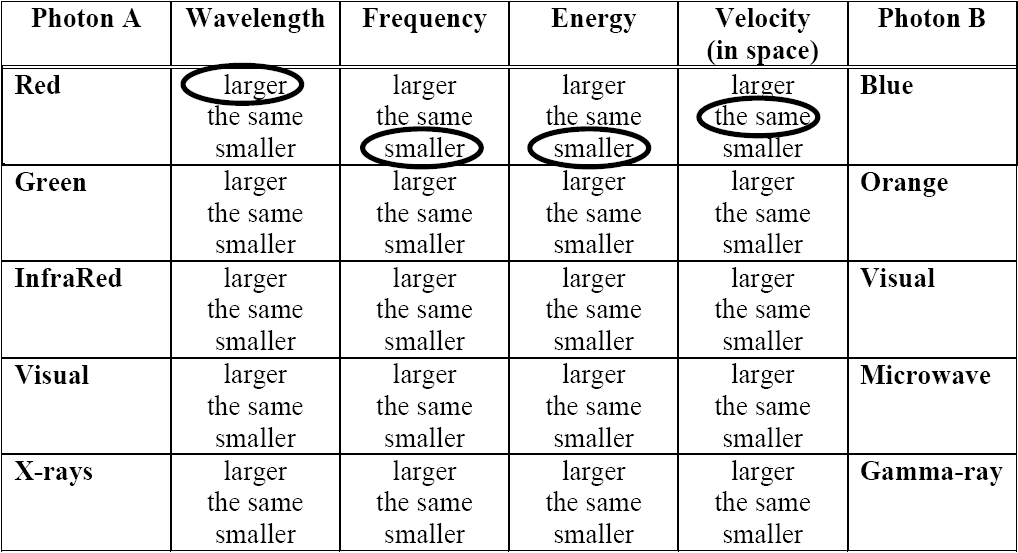
When one variable increases at the same time that another variable increases scientists say that the two variables are directly proportional. A graph of such a pair of variables would be a straight line. When one variable decreases in proportion to the increase of another variable then the relationship is described as inversely proportional. The graph of such a relationship is an inverse curve that looks like y = 1/x.
Use what you already know about light and the results of your work on the table comparing photons to answer the following questions.
For each energy level of an atom there are three energy ranges for photons that interact with the atom.
| Wavelength | Frequency | Energy | Transition | Region |
| 122 nm | 2.5 × 1015 Hz | 10.2 eV | 1 --> 2 | UV |
| 1 --> 3 | ||||
| 1 --> 4 | ||||
| 1 --> 5 | ||||
| 1 --> 6 |
| Wavelength | Frequency | Energy | Transition | Region |
| 2 --> 3 | ||||
| 2 --> 4 | ||||
| 2 --> 5 | ||||
| 2 --> 6 |