By doing this lab the student will learn how to operate a bunsen burner safely. Also, the student will explore and try to explain an apparent violation of the Law of Conservation of Matter. This is an inquiry lab in which students must design and carry out experiments to complete the objectives.
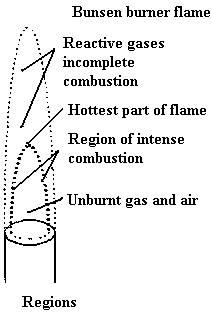
The bunsen burner is an extremely convenient source of heat for the chemical laboratory. It was designed by Robert Bunsen more than 100 years ago when he was a researcher at the University of Heidelberg in Germany. He needed a flame that was steady, clean and hot and these are all things that ordinary yellow flames are not. Ordinary flames are sooty and burn at low temperatures. Not to mention how much they flicker. Bunsen’s burner burns blue and very hot because it is designed to completely combust the gas without producing soot. It can do this because it mixes the gaseous fuel with air before it starts to burn.
The burner you will use may have two valves. One of them controls the amount of fuel delivered to the barrel and the other controls the amount of air. If you have a different type of burner then there is only one control built into the barrel. The sliding perforated sleeve controls the amount of air that the fuel mixes with. In this lab you will learn how to operate either type of burner properly.
You will learn how to light and maintain a hot, blue, noisy flame. You will learn the difference between a luminescent flame and one in which soot and light is reduced to a minimum in order to maximize heat. You will learn about the conservation of matter in physical changes such as when popcorn pops.

There are three objectives in this lab. You must work in pairs to complete each objective. Before moving on to another section of the lab you are required to obtain your teacher’s initials. Points will be deducted for failing to do so.
While you are working use the data you collect to decide whether you are on the right track. Modify future experiments based on the data you collect. Be reflective and think about what your results mean before proceeding. Do not work to just get it all done as quickly as possible. Finally, be creative! Solving problems in science requires a different kind of creativity from that needed to create art or music but it is just as important in science as in the arts.
In this objective you must simply light and correctly adjust the flame of a bunsen burner. All individual students must demonstrate their proficiency in this skill.
In this objective you must establish whether mass is gained, lost, or stays the same when you pop popcorn using the heat of the bunsen burner. You must be able to report how much mass is lost/gained per kernel and what percentage this is of the original mass.
In this objective you must design one or two experiments which could explain why the popcorn gained/lost mass in the experiments you performed in Objective Two. If it is possible to carry out your experiment(s) in the lab, your teacher will advise you on doing so. At no time should you perform experiments not previously authorized by your teacher.
Remember to record your observations in your lab notebook or on a piece of paper in your binder before you leave class. When making observations be sure to use all of your senses except taste. Never taste anything in the chemistry lab. Even if it looks edible.

When you have completed your work in this section check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can move on to the next objective. Initials will be given for successfully demonstrating that you can light and correctly adjust the bunsen burner.
Answer the questions below before moving on to Objective Two.
When you have completed this objective check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can move on to the next objective.
Is mass conserved in the popping of popcorn? Scientists say it is but what do your measurements say? Pop some and find out.
Your teacher will demonstrate a method to use the heat from the burner to pop popcorn. You should practice with a few kernels before popping any as part of your experiments. It may take time to perfect your technique as you may need to adjust the height of the ring or the flame characteristics. Some suggested data to collect:
Design your own data table for this part of the lab. Be sure to plan carefully and leave room for multiple trials of the same experimental set-up, if needed. Also, remember to leave room to enter average values, changes in mass and percent changes. You may find it helpful to use a ruler. Sketch your data table on a separate piece of paper and show it to your teacher for approval before putting the final version here. You must obtain your teacher’s initials before putting your data table here.
Answer the questions below before moving on to Objective Three.
Change in Mass (Δm)
Δm = Masspopped – Massunpopped
Percent Change in Mass
Δm
————————————————————————— × 100%
Massunpopped
When you have completed this objective check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can move on to the next objective.
In this objective your job is to design an experiment to test your answers to the last two questions from Objective Two. Your experiment(s) must result in data that explain the change in mass of the popcorn kernels. Also, your data must show whether mass is gained from nowhere, gained from an identifiable source, actually destroyed, or is simply difficult to measure. If there is time, and if your experiments are practical, you will be given a chance to carry them out. You need to fill out this section whether or not there is time to attempt the experiments in the lab!
Design your own data table for this part of the lab. Be sure to plan carefully and leave room for multiple trials of the same experimental set-up, if needed. Also, remember to leave room to enter average values, changes in mass and percent changes. You may find it helpful to use a ruler. Sketch your data table on a separate piece of paper and show it to your teacher for approval before putting the final version here. You must obtain your teacher’s initials before putting your data table here.
Answer the questions below using your lab data, if possible. If you could not carry out any experiments for this objective then use your observations from the previous objective to come up with a reasonable answer.
When you have completed this objective check in with your teacher. This is a required part of the lab and your teacher’s initials are required before you can consider this lab finished.
This section is optional but is worth 7 points out of 100 for the whole lab. Turn in your lab as soon as you have your teacher’s initials if you do not intend to do it. If you did everything else well then you can afford not to earn these points. On the other hand, you can use these 7 points to help bring up your overall average…
Research the origins of the establishment of the law of conservation of matter. Look up Antoine-Laurent de Lavoisier (Antoine Lavoisier) and his wife Marie-Anne Pierrette Paulze. The following web sites are a good place to start:
http://en.wikipedia.org/wiki/Antoine_Lavoisier (especially the section on stoichiometry)The assignment: find at least one other source, preferably a book. Read about Lavoisier. Write two or three paragraphs about his life. Include one paragraph about his work which established the principle of the law of conservation of matter. The point of this research is to understand the origins of the law of conservation of matter. Make it clear in your writing that you understand the law and how Lavoisier gathered evidence for its truth.