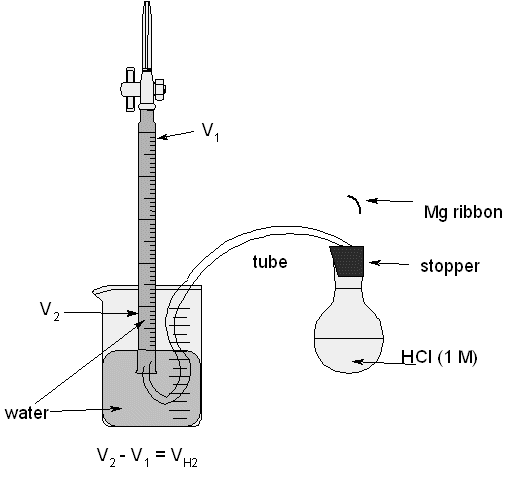
| 1. a standard 50 mL buret | 6. sufficient water to fill beaker and buret |
| 2. clamp, buret clamp and stand | 7. 100 mL round-bottom flask |
| 3. medium beaker (≥250 mL) | 8. rubber tube and 1-hole stopper |
| 4. 0.02 to 0.04 g Mg ribbon | 9. 100 mL graduated cylinder |
| 5. 50 mL HCl (1 M) | 10. steel wool |
