Lab: Identifying Elements
Introduction
In this lab you will have access to a set of samples of elements. Each sample is exactly one mole of that element. You will identify each element in the set by making measurements. Your identification must rest on two pieces of quantitative evidence. Data on atomic mass and density of the elements is provided for your reference.
Procedure
Use tools such as lab balances, rulers, and graduated cylinders to make measurements you can use to identify the element samples. Create a data table for your data and calculated results. If you are not sure what data to collect consider looking at the post-lab for a hint.
Element Data Table 1
organized by atomic number
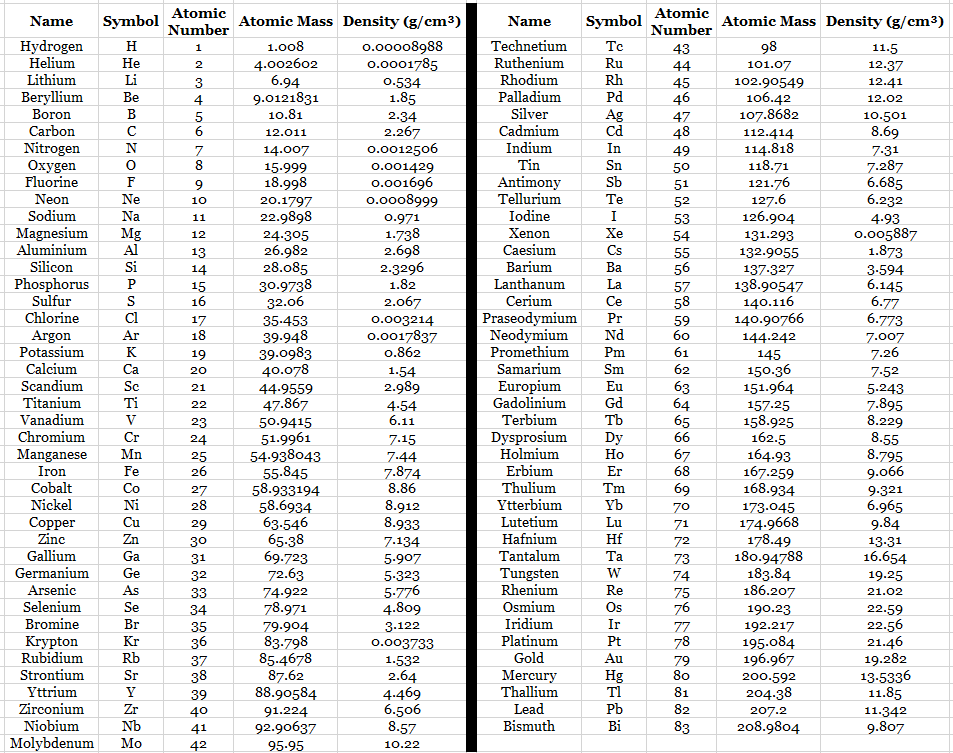
Element Data Table 2
organized by density
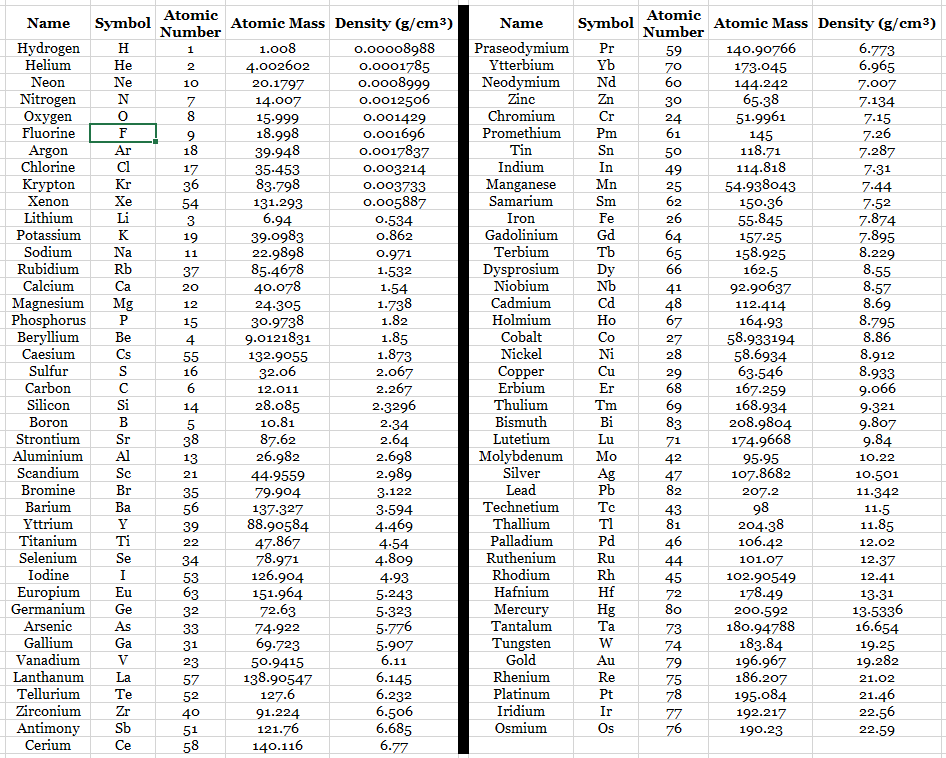
Post-lab
Answer the following questions in a typed document. Do your own work individually.
- Create and format a data table including these headings: Sample ID, Mass, Volume, Density, Molar Mass, Element Name/Symbol. You should have all six samples in your data table. Headings should be bold and all cells should have borders.
- How did your knowledge that each sample had exactly one mole of an element help you in determining the elements’ identities?
- Which element has the heaviest atoms? Which element has the lightest atoms? Explain how you know in each case.
- Atoms also have different volumes. Since the samples all have the same number of atoms you can make an educated guess about the volumes of their atoms. Put the samples in order by volume and discuss any circumstances where you cannot easily distinguish between elements.
- Each element’s molar mass is the mass in grams of one mole of the atoms of that element. Make a table to contain the results of the following calculations:
- Divide the molar mass of each element by Avogadro’s Number (6.02 × 1023) to find the mass of one atom of the element in grams. Report results using scientific notation.
- Convert the mass in grams of one atom of the element to atomic mass units (amu) using the conversion factor, 1 g = 6.02 × 1023 amu.
- Why are the molar mass and the mass of one atom the same number, though with different units, for all of the elements?
- Explain how you can use the mass of a sample and the molar mass of the material it’s made of to count the atoms in the material.