It is one of the most famous experiments of all time. More than 25 years after conducting the experiment, Ernest Rutherford described the results this way:
It was about as credible as if you had fired a 15-inch shell at a piece of tissue paper and it came back and hit you.The experiment itself was actually the culmination of a series of experiments, carried out over a five-year period, dealing with the scattering of high-energy alpha particles by various substances. What is “Rutherford Scattering” and why was it so important?
Background
Ernest Rutherford received the Nobel Prize in Chemistry in 1908 for his investigations into the disintegrations of the elements as a result of radioactive decay. Among the products of the radioactive decay of elements are alpha particles—small, positively charged, high-energy particles. Alpha particles are in fact completely ionized 4 2He nuclei. In trying to learn more about the nature of alpha particles, Rutherford and his co-workers, Hans Geiger and Ernest Marsden, began styding what happened when a narrow beam of alpha particles was directed at a thin peice of metal foil. Alpha particles are a type of nuclear radiation, traveling at about 1/10 the speed of light. As expected for such high-energy particles, most of the particles penetrated the thin metal foil and were detected on the other side. What was unexpected was that a few—a very few, to be sure—of the alpha particles were actually reflected back toward the source, having been “scattered” or had their paths bent due to their encounters with the metal atoms in the foil target. The number of alpha particles that were reflected back depended on the atomic mass of the metal. Gold atoms, having the highest atomic mass of the metals studied, gave the largest amount of so-called “back scattering”.
Rutherford’s scattering experiments
No instrument allows the atom’s nucleus to be observed directly, so scientists have to find other ways to learn about it. Scientists at Jefferson Lab in Virginia use a machine called an electron accelerator to probe atomic nuclei. This accelerator takes electrons, forms them into a beam about the width of a human hair and speeds them up to nearly the speed of light. This fast moving beam of electrons is then directed at a target. Some of the electrons in the beam interact with some of the atoms in the target. When this occurs, the electron changes direction and some particles could fly out of the nucleus. Detectors placed around the target record the paths of the electron and nuclear fragments. These collisions provide scientists with clues about the structure of the nucleus. By bouncing a marble off of a hidden target, you simulate the experiments done at particle accelerator laboratories like Jefferson Lab.
Procedure
Prepare your lab notebook by putting the title of the lab and the date at the top of the first empty page in your lab notebook. Remember to make an entry in the table of contents and to number the pages as you go along. When writing in your lab notebook you should always say what you did, what you observed and what it means. See the Data section for a hint about how to organize the material in your lab notebook for this lab.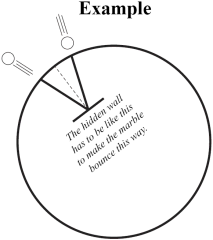
Data
Questions
To Turn In
For this lab turn in a sheet of paper with your data tables neatly transcribed onto them.