Making a Silver Mirror
Introduction
In this lab students will coat the inside of a small test tube with metallic silver, making a shiny mirror in a dramatic transformation. This procedure creates a keepsake for the student and demonstrates some interesting chemistry. Silver compounds undergo interesting transformations: a clear solution is mixed with another clear solution, producing a dark gray powder. Another clear solution is added, which redissolves the powder. When a glucose solution is added the solution turns dark again and after a few moments the inner surface of the test tube turns bright with deposited silver metal.

|
|
Figure 1(1) It is well known that crows and other corvids like shiny things. It may not be true, but it is well known. |
|
|
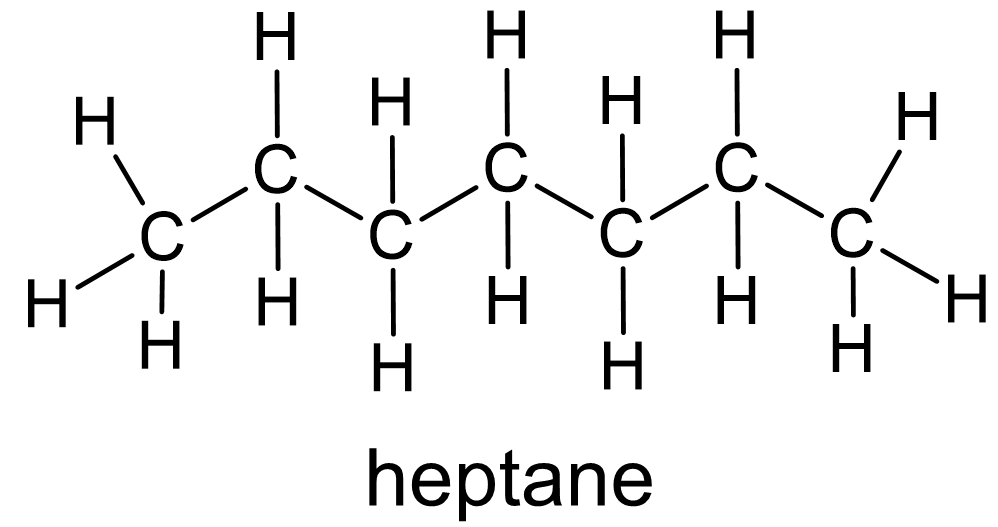
Figure 2 Alkanes have only single bonds between carbon atoms. |
|
|
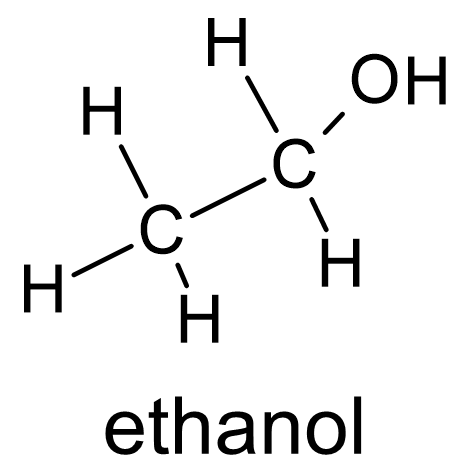
Figure 3 Alcohols have oxygen singly bonded to a carbon atom and a hydrogen atom. |
|
|
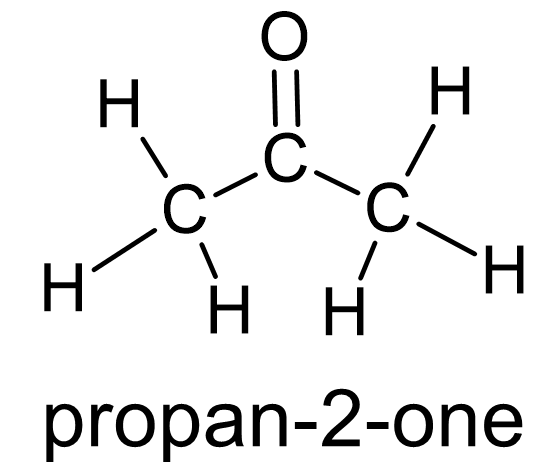
Figure 4 Ketones have oxygen doubly bonded to a carbon atom which itself is bonded to two other carbon atoms. |
|
|
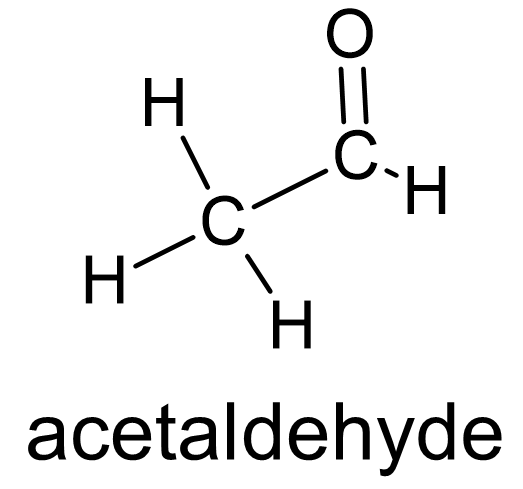
Figure 5 Aldehydes have oxygen doubly bonded to a carbon atom which itself is bonded to one carbon atom and one hydrogen atom. |
|
|
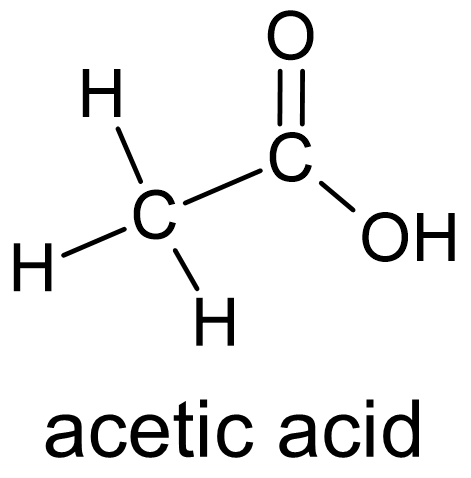
Figure 6 Carboxylic acids have two oxygen atoms bonded to the same carbon atom. |
Organic Functional Groups
To begin, organic, or carbon-based, molecules are classified and understood based on their wide variety of structures. Identifiable parts of organic compound structures are called functional groups. A carbon chain with only single bonds between the carbon atoms is called an alkane (fig. 2). This is the simplest functional group. Such molecules are relatively unreactive. They are mostly burned as fuels.
More interesting molecules incorporate other atoms into their structures, such as oxygen. Oxygen atoms play a central role in several organic functional groups. In alcohols (fig. 3) an oxygen atom has a single bond to a hydrogen atom and a carbon atom. Alcohols such as ethanol make excellent solvents that lie on a spectrum between a polar solvent, like water, and non-polar solvents. Ethanol is the kind of alcohol in beverages such as beer and wine. Sugar molecules have an alcohol group, also known as a hydroxyl group (—OH), attached to nearly every carbon atom in a chain.
Ketones are another functional group involving oxygen. Their structure involves a double bond between carbon and oxygen C⚌O) known as a carbonyl group. In ketones, the carbon atom of the carbonyl group is bonded to two other carbon atoms (fig. 4). Aldehydes also include a carbonyl group with the difference being that the carbon atom is bonded to one carbon atom and a hydrogen atom (fig. 5). Sugars are a large class of related molecules and in some their structure includes a ketone or an aldehyde. Sugars which include a ketone group are called ketoses and sugars which include an aldehyde group are called aldoses.
When oxygen is added to an aldehyde in a chemical reaction it is transformed into a carboxylic acid. A carboxylic acid is a functional group with a carbonyl carbon bonded to one carbon atom and one hydroxyl group (fig. 6). It appears to be an aldehyde in which —OH has replaced —H.
The Tollens Test
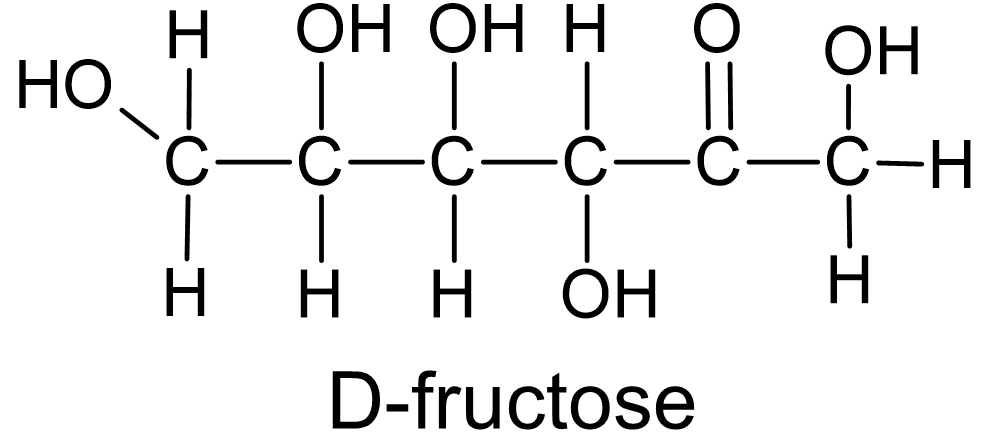
As part of determining the structure of various sugars it was important to distinguish whether the sugar molecule included an aldehyde group or a ketone group. The aldoses are called reducing sugars because they are capable of reducing ions or molecules in the process of becoming oxidized and transformed into carboxylic acids. A common sugar which falls into this category is glucose (also known as dextrose, fig. 8). Non-reducing sugars, or ketoses, include a ketone group instead of an aldehyde. One example is table sugar, or sucrose, and another is fructose (fig. 9).
In the silver coating reaction an aldose sugar is oxidized into a carboxylic acid. The original purpose of the reaction was to detect qualitatively whether a sugar was an aldose, which would cause the silver to appear, or a ketose, which would not. It was published by the German chemist Bernhard Tollens (1841 - 1918) in 1882 and is widely known as Tollens Test. (2)
Modern chemistry laboratories no longer need this test to help to determine the molecular structure of sugars. Instead a variety of methods can be used: Nuclear Magnetic Resonance (NMR) spectrometry, infrared spectrometry, gas chromatography, and mass spectrometry. But at the time Tollens published his investigations of reactions between silver and sugar molecules, none of these technologies had been invented. As a result, the ability to tell with a quick addition of readily available chemicals whether a sugar included an aldehyde group was very useful. In modern times it provides an opportunity to learn about the underlying chemistry.
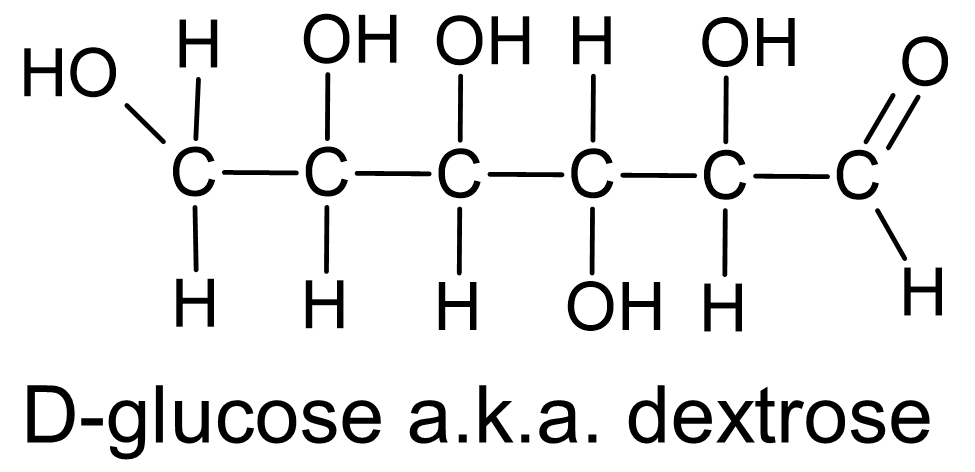
|
|
Figure 8 Sugar molecules which include an aldehyde group in their structure are reducing sugars. Also known as aldoses. |
|
|
Figure 9 Sugar molecules which includes an ketone group in their structure are known as ketoses. |
Reactions of Silver Ions
Silver is a well-known metal used in jewelry and to make mirrors. The shiny metal is in a state known to chemists as ‘reduced’. Metals form positive ions in compounds and when in ionic form they make crystalline ionic compounds which may dissolve in water. Reduction is the process by which an ion, atom, or molecule gains one or more electrons. In the case of metals this means that they are transformed from ionic form to neutral atoms, or metallic form.
Equation 1 Ag+ + 1e– → Ag
Equation 1 shows the reduction of a silver ion, which has a +1 charge, to become a neutral silver atom. This is a so-called half-reaction, showing only the reduction. The process of oxidation is when an atom, ion, or molecule loses one or more electrons. All reduction half-reactions must be coupled with an oxidation to provide the electron(s). In the reaction that creates the silver mirror the aldose sugar molecule glucose provides the electron.
In order to create a mirror, however, it is not sufficient to mix solutions of silver ions and glucose. When those two substances are mixed the result is a fine powdered form of metallic silver which settles to the bottom of the test tube. No mirror forms. The problem is that the reaction is too fast: the silver ions do not have enough time to find their way to the wall of the test tube before they are reduced to metallic form. Because of this, some additional steps are required.
The first step of the procedure is to add sodium hydroxide solution. The reduction of silver by glucose works best in a solution that is strongly basic, with a high concentration of hydroxide ions (OH–). Silver ions cannot remain dissolved in the solution when the pH is too high and so the silver oxide precipitate forms when we add the sodium hydroxide (Equation 2).
Equation 2 2AgNO3(aq) + 2NaOH(aq) → Ag2O(s) + H2O(l) + 2NaNO3(aq)
This silver compound traps all of the silver ions in the solid phase, meaning that they are no longer available to be reduced on the glass to make the mirror. In order to make the silver ions available again for the reduction reaction another step is required.
The second step of the procedure is to add strong ammonia (NH3) solution. The silver ions form a complex ion with the ammonia molecules with the formula Ag(NH3)2+ (Equation 3).
Equation 3 Ag2O(s) + 4NH3(aq) + H2O(l) → 2Ag(NH3)2+(aq) + 2OH–(aq)
Complex ions are combinations of metal ions with negative ions or neutral molecules which may be more soluble in water than the metal ion alone. By forming bonds with the ammonia molecules the silver ions redissolve and the gray silver oxide precipitate disappears. The resulting complex ion has the formula Ag(NH3)2+ and is called diamminesilver(I). Besides redissolving the silver ions, the formation of the complex ion has another benefit. The silver ions alone reduce too easily, forming a metallic precipitate. The complex ion, on the other hand, is harder to reduce(3) and so it requires contact with the test tube walls for the reaction to take place (Equations 4a and 4b). The numbers come from the study of electrochemistry and thermodynamics and they indicate that the reduction of plain silver ions is more spontaneous than the reduction of the silver-ammine complex. If this means nothing to you, you can safely ignore it.
Equation 4a Ag+ + 1e– → Ag E° = +0.799 V Equation 4b Ag(NH3)2+ + 1e– → Ag + 2NH3 E° = +0.373 V (3)
Once the solution is strongly basic and the silver ions are returned to their dissolved state by forming a complex-ion, the sugar will be added. The sugar used is glucose (also called dextrose), which is an aldose sugar. Aldehyde groups in organic molecules are good at providing electrons to reduce ions or other molecules. When they do so, the aldehyde group is transformed into an organic acid group. Sucrose, or table sugar, is a ketose and so would have no effect. The overall reaction is given here as Equation 5.
Equation 5 C6H12O6 + 2Ag(NH3)+ + 3OH– → C6H11O7– + 2Ag + 4NH3 + 2H2O
Pre-lab Questions
Answer these questions before coming in to the lab. If you do not know how to make the drawing or answer the question then that is a hint that you should go find out. Study the figures in the text for help and inspiration. Most of what you need to know to answer these questions can be found in the introductory text in this lab handout. If you do outside research, cite your sources, make sure they are legitimate, and do not copy and paste. Read and understand and write in your own words, always.
- Draw Lewis structures of molecules with each of the following organic functional groups. You may use this lab handout or other resources to find appropriate molecules.
- alkane
- alcohol
- ketone
- aldehyde
- carboxylic acid
- What kind of organic functional group is the Tollens Test designed to detect? What is the effect on this functional group after it has done its work? That is, how is it transformed and what new functional group comes to replace it?
- What is the chemical process of reduction? How does it apply to the silver in this lab experiment?
- What chemical is oxidized in this experiment? Explain.
- Why does the solution have to be strongly basic for the experiment to work? What effect does this high pH have on the solubility of silver?
- What is a metal complex ion and how does it play a role in this experiment?
Materials
- 600-mL beaker
- warm water bath at 40°C - 50°C
- lab goggles
- 1 test tube (vol. 14 mL, 9 cm × 1.4 cm)
- size 0 rubber stopper
- wash bottle
- 1 mL of 6 M sodium hydroxide (NaOH)
- 20 drops of 0.42 M silver nitrate (AgNO3)
- 20 drops of 1.67 M sodium hydroxide (NaOH)
- 20 - 26 drops of 6 M ammonia (NH3)
- 20 drops of 0.67 M dextrose (C6H12O6)
Safety
- If you choose not to wear safety glasses you are choosing to sit out the lab.
- Sodium hydroxide is a strong base and can cause severe damage to skin and eyes. Eye exposure can lead to blindness if not addressed immediately. Avoid contact and wash hands after handling it. If in eyes, rinse with cold water as soon as possible for up to 15 minutes.
- Ammonia solution is strongly alkaline and corrosive and can cause severe damage to skin and eyes. Eye exposure can lead to blindness if not addressed immediately. Avoid contact and wash hands after handling it. If in eyes, rinse with cold water as soon as possible for up to 15 minutes.
- Silver nitrate solution is toxic and corrosive. Avoid contact with skin and eyes. Wash hands after handling. If in eyes, rinse with cold water as soon as possible for up to 15 minutes.
- The solution left after the mirror has formed contains very small amounts of toxic silver ions and may, if left to dry, lead to the formation of potentially explosive compounds silver nitride(4) or silver fulminate(5). It must be diluted 20-fold and poured down the drain.
- Always close containers of chemical supplies when they are not in use.
- Wash hands thoroughly with soap and water before leaving the laboratory.
Procedure (3) (4) (5) (6)
Be aware of the chemical hazards as you follow this procedure. If done carefully there is little chance of harm.
- Fill a 600-mL beaker with about 500 mL of water and set it nearby. This water is meant to dilute and dispose of the solution once your mirror has been created.
- Locate the warm water bath prepared by your teacher. This should be a 600-mL or 1-L beaker filled with water on an electric hot plate. Check the thermometer in the water bath to ensure the temperature is between 45°C and 55°C. In this temperature range it is safe to submerge your fingers.
- Optional: Obtain a new test tube and weigh it on an analytical balance to the nearest 0.0001 g if your balance supports this level of precision. After the experiment, and after the test tube is completely dry, weigh it again to find out how much silver was actually added to the inner surface of the glass.
- Whether you weigh the test tube or not, you will need to clean the inner surface. To do so, add about 1 mL of 6 M sodium hydroxide (NaOH), insert a size zero stopper, and shake for about one minute. Remove the stopper and rinse three times with fresh water, being careful not to get the solution on your fingers.
- Add 20 drops of the 0.42 M silver nitrate (AgNO3) solution to the test tube. One drop is about 0.06 mL so this will be a volume of about 1.2 mL.
- Add about 20 drops of the 1.67 M sodium hydroxide (NaOH) solution to the test tube. Observe that this causes a precipitation reaction. The gray powder that forms is mainly silver oxide (Ag2O), which is insoluble in water. Watch the reaction closely and add sodium hydroxide solution until adding a drop no longer produces a cloud of gray powder.
- Add about 20 drops of the 6 M ammonia (NH3) solution to the test tube. Swirl and mix the solution until the precipitate (the gray powder) has completely redissolved. You may insert the stopper and shake to improve mixing. In this step the silver-ammonia complex necessary for the mirror reaction is formed and all of the silver oxide needs to be reacted. You may need to add a few additional drops to dissolve all of the silver oxide. Continue adding 1 or 2 drops at a time until when you mix the solution there is no more solid swirling around. Keep track and tell your teacher how many drops you used.
- Add 20 drops of the 1.67 M dextrose (C6H12O6) solution.
- Use a wash bottle to add water to the test tube to fill it to within about 1 cm of the top. Leave room to insert the stopper.
- Insert the size zero stopper and be aware that the air pressure inside it will increase and that the stopper will tend to come loose again. Once you feel you have it secure, hold the test tube in the warm water bath.
- Hold the test tube submerged in the warm water bath to heat it up. This speeds up the reaction considerably. You may do this by hand or you may use a pair of test tube tongs. Take it out to shake it every so often. After a short time (1 - 2 min) you should see the formation of a silver mirror inside the test tube. Keep it submerged in the warm water for a total of about 5 min and continue shaking periodically so that any remaining silver has a chance to also be added to your mirror.
- The solution that is in the test tube can, if left out, lead to the formation of dangerous compounds, which can explode, but not in a fun way. Pour the contents of your test tube into the beaker of water you filled in the first step. Then turn on the cold water in the sink and empty the large beaker into the flowing water. Let water run for 30 seconds or so to dilute and wash all of the material down the drain.
- Use the wash bottle to rinse the test tube with small amounts of water, gently so you don’t disturb the silver coating. Rinse 3 or 4 times.
If desired the test tube can be used as a small vase for dried or fake flowers but be aware that this may scratch the mirror’s surface. Do not add water to the test tube or this may ruin the silver, too. If you wish to use it for live flowers with water or if you want to protect the surface you will have to find a waterproof finish for the interior surface. Clear nail polish may work but the author has not tested this suggestion.
Another way to display the silvered test tube is to glue the stopper into the top and to add a hook to make it into an ornament.
Post-lab
For fun, answer the following calculation questions.
- What is the thickness of the layer of silver atoms in picometers (pm)? Given: the surface area that is covered by silver is about 42 cm2, the number of silver atoms is 5 × 10–4 mol, the radius of a silver atom is 160 pm, the volume of a sphere is V = 4/3πr3, and the total volume of all of the atoms is approximately the number of atoms times the volume of one atom. Hint: Divide the total volume of the atoms by the surface area to find the thickness.
- How many atoms thick is the layer of silver given that a silver atom has a diameter of 320 pm?


