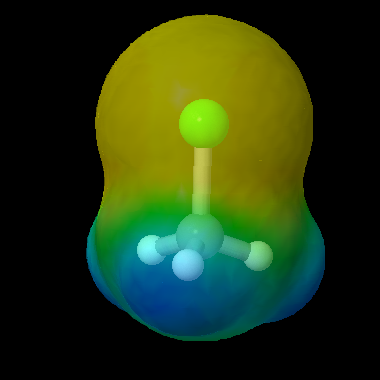
This molecule has a dipole with a slight separation of charge between the carbon atom and the chlorine atom. The yellow color of the electron cloud around chlorine shows the higher electron density at that end of the molecule.
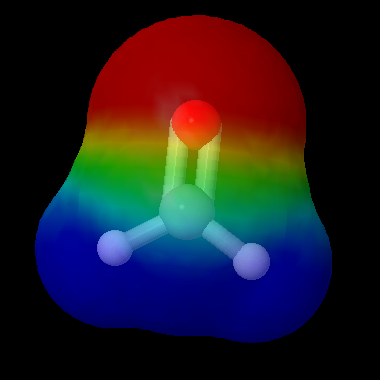
Formaldehyde has a stronger dipole than chloromethane. The red color on the oxygen atom and the blue color on the carbon atom illustrate this. The electron density is higher at the oxygen end of the molecule. Molecules which contain a C=O double-bond are called carbonyls; such molecules are both electrophiles (the carbon atom will bind to another molecule with an electron pair to donate) and nucleophiles (the oxygen atom will donate a lone pair to bind to an electron-deficient molecule).
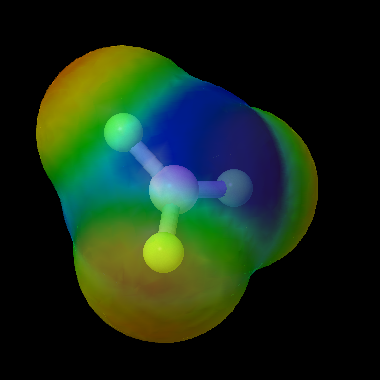
This symmetrical molecule does not have a dipole but it does have an unequal distribution of electron density. The central boron atom in this molecule is electron deficient so this molecule is a Lewis acid. Another term for this is electrophile, which means that the molecule has a tendency to bind to atoms in other molecules with high electron density.
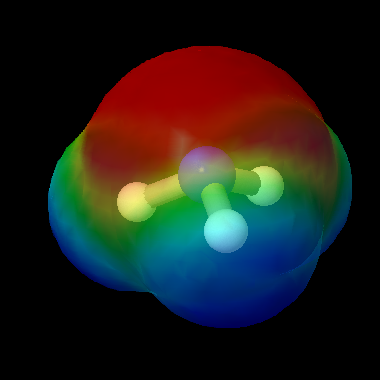
Ammonia is a polar molecule. The lone pair on the nitrogen atom and the concentration of electron density (as shown in this map) mean that ammonia is a Lewis base. Another term for this is nucleophile, which means that the molecule will bind to atoms in other molecules with a slight positive charge.