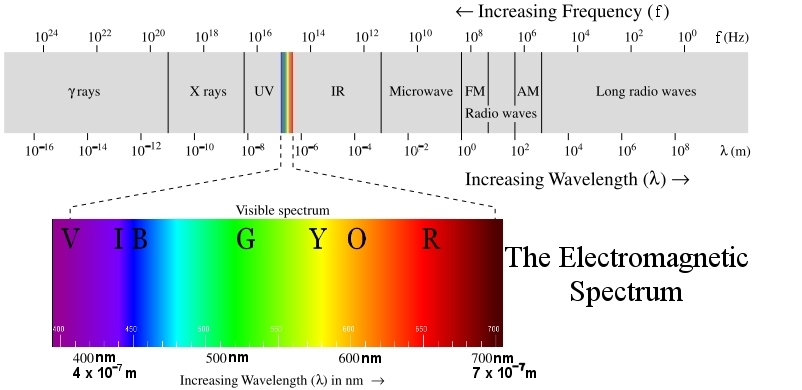
 λ stands for wavelength in meters (m)
λ stands for wavelength in meters (m)
|
||||||||
| Important Formulas |
c = λf
(relates frequency and wavelength of light) |
|||||||
|
E = hf
(relates energy of one photon and frequency of light) |
||||||||
| Important Conversions | ||||||||
| micrometers (μm): 1 m = 1 × 106 μm | ||||||||
| nanometers (nm): 1 m = 1 × 109 nm | ||||||||
| gigahertz (GHz): 1 GHz = 1 × 109 Hz | ||||||||
| megahertz (MHz): 1 MHz = 1 × 106 Hz | ||||||||
| kilohertz (kHz): 1 kHz = 1,000 Hz | ||||||||
|
electron volts (eV) and joules (J): 1 eV = 1.602 × 10–19 J |
||||||||