The following paragraphs are meant to summarize the activity you did in class. Compare this summary to the one your teacher asked you to write. For homework use your comparison to write down all changes you would need to make to your summary to make it accurate and complete. If you notice something that this summary leaves out then please bring it to the attention of your teacher. Your changes list should be typed and handed in to earn credit for this assignment.
In this activity we learned how to draw the ground state orbital diagram for the first 18 elements. We also learned how to write the ground state electron configuration for the same elements.
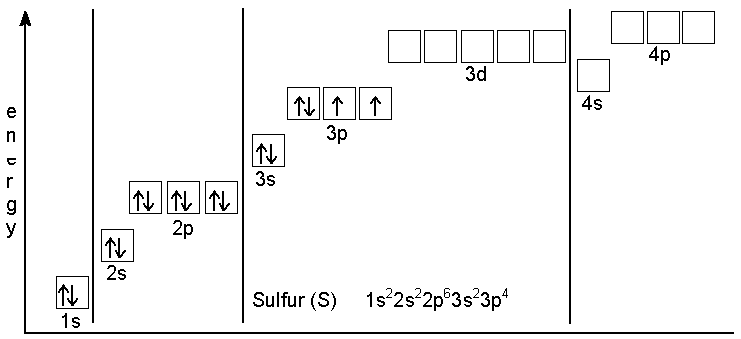
Electrons occupy orbitals, or regions of space where they are most likely to be found. These orbitals are organized into levels with different amounts of potential energy. Within each level there can be sub-levels that also differ in the amount of potential energy an electron has when in those levels. Each level has a number (1, 2, 3,…) and each higher number is at higher potential energy than the last. In this activity there were two types of sub-level: s- and p-. An s-sub-level can hold two electrons in one orbital and is slightly lower in energy than the p-orbitals in the same level. A p-sub-level has three orbitals which can hold a total of 6 electrons, or two each. Within an energy level all p-orbitals are at the same potential energy. Electrons have a characteristic called spin and they can either be spin-up (counter-clockwise) or spin-down (clockwise). They do not literally spin on an axis like the planet Earth but they do have a tiny magnetic field that would exist if an electrically charge particle were spinning. This is shown in diagrams with arrows that point up or down.
When filling an orbital diagram or writing a ground state electron configuration there are three rules to keep in mind. First is the Aufbau Principle which requires that electrons fill lower levels completely before any electrons enter a higher level. Also, it requires that within a level the s-sub-level must be filled before any electrons enter the p-orbitals. The second rule is the Pauli Exclusion Principle which says that two electrons in the same orbital have to have opposite spins and that only two electrons can be found in any single orbital. Third is Hund’s Rule which requires that electrons in a diagram must be added in so that all orbitals in a sub-level have one electron (and all with the same spin) before any other electrons are placed in the same sub-level. For example, nitrogen has three electrons to place in the 2p orbitals. Instead of having two electrons in the first orbital and one electron in another orbital Hund’s Rule requires that all three electrons be placed in separate orbitals and all with either up or down spin.
The written-out electron configuration for the ground state of an atom looks like this: 1s22s22p63s23p4 for the element sulfur (S), which is number 16 in the periodic table and has 16 electrons. Each orbital is shown in the list as a number and a letter followed by a number which is smaller and above the rest. The first number gives the energy level’s number. The letter gives the type of sub-level and the small numbers represent how many electrons are in that orbital or set of orbitals. In the example orbital diagram below it is clear that the electrons in the 3p sub-level have been placed as spread-out as possible according to Hund’s Rule.