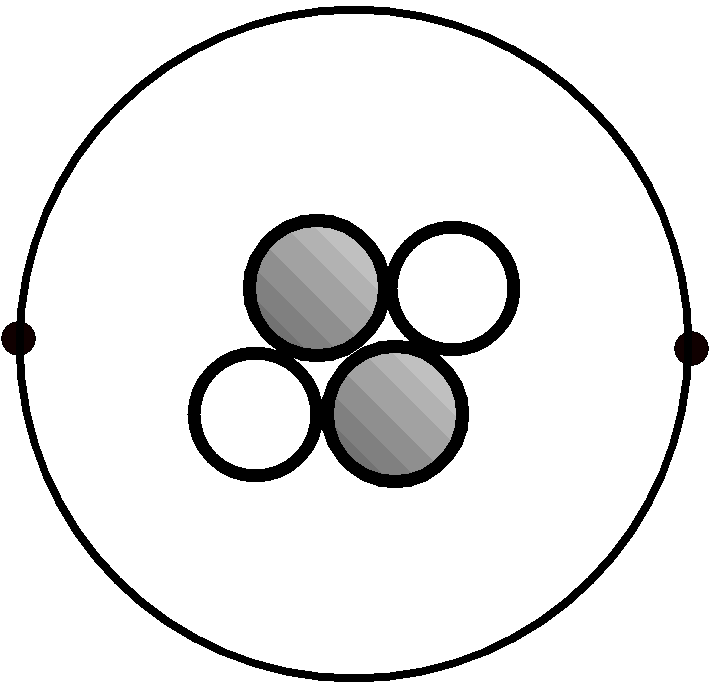
A model of a helium atom.
Protons are large and shaded, neutrons are large and white, electrons are small and black.
Atoms are the building blocks of all matter. Everything we can see around us is made of atoms: your chair, the sun, the stars, your lunch, even you. In one sense, atoms are the smallest pieces of ordinary matter that there are. The idea of a ‘smallest piece of matter’ has been around for at least 2,600 years. Around 600 b.c. a Greek philosopher by the name of Democritus proposed the existence of atoms. He suggested that if you were to cut something in half, then cut it in half again and again and again you would eventually find that there was an end to the process. You would come to a point where you could not cut what you have left in half anymore. He called what was left an atom. The word ‘atom’ comes from Greek for ‘uncuttable’.
Another Greek philosopher by the name of Aristotle disagreed. He claimed that there was no such thing as a smallest piece of matter. Aristotle believed that you could go on cutting things in half forever. The two philosophers never tried to do any experiments to find out who was right and the world waited until the middle of the 18th century to find out (around 24 centuries later!). The ancient Greeks did not believe in doing experiments to find out about the world. Instead, they tried to make discoveries simply by thinking carefully about things.
As it turns out, neither of them was exactly right or exactly wrong. Democritus’s idea of the atom is strikingly similar to the modern idea of an atom. He was wrong about one thing, though: an atom is made up of smaller parts and is divisible after all. Aristotle believed matter was perfectly continuous and fundamentally had no smallest part. In a way, he was partly right about one thing: atoms are not the smallest pieces of matter. Atoms have parts called protons (p+), neutrons (n0) and electrons (e-). There really are no smaller parts of electrons but protons and neutrons both have smaller particles that make them up. That is a discussion for another day, though.
Protons (p+) are particles that are found in the nucleus of an atom. The nucleus is the tiny, massive center of an atom. All protons have an electric charge of +1 and a mass of 1 amu. An atomic mass unit (amu) is the unit used to describe the masses of atoms and parts of atoms.
Neutrons (n0) are bound tightly to protons in the nucleus. They add mass to the nucleus and perform an important function in keeping the nucleus from falling apart. All neutrons have an electric charge of zero (0). This is where they get their name since the word neutral is used to describe items with zero electric charge (neutral - neutron). All neutrons have a mass of 1 amu.
Electrons (e–) orbit around the nucleus at a great distance from it. They all have an electric charge of –1 and a mass 1836 times smaller than the mass of a proton: 5.4 × 10-4 amu. Negative charges are attracted to positive charges and this is why the electrons orbit the positively charged nucleus. They do not simply fall onto the protons in the nucleus for reasons that are too complex for this introduction.

|
|
Figure 1
A model of a helium atom. Protons are large and shaded, neutrons are large and white, electrons are small and black. |
The nucleus of an atom is a small, very dense object where all the protons and neutrons in an atom can be found. More than 99% of the mass of an atom is in the nucleus. Looked at as a particle in its own right, the nucleus is positively charged and can have a variety of different masses.
The amount of positive charge in a nucleus depends only on how many protons are part of it. The number of protons in a nucleus is called the atomic number. It is unique for every element and serves as the identifying feature. Each element in the periodic table is defined by the number of protons that are in the nuclei of its atoms. The atomic number of the atom shown in fig. 1 is 2, making it an atom of the element helium (He). In order to talk about the idea of atomic number without spelling out those two words every time, chemists use a symbol. The letter Z is used to symbolize atomic number. Atomic numbers are found in each and every box on the Periodic Table of Elements above the letter-symbol for the element.
In addition to an atomic number, every element has a unique atomic symbol, one or two letters that stand for an element. Normally, the symbol is derived from the name: Carbon is C, Oxygen is O, Boron is B, Uranium is U, etc. Some symbols are derived from Latin names for elements: Silver (Ag) gets its symbol from the Latin word for silver: Argentum. Gold’s symbol (Au) is from Latin, too: Aurum. For every symbol the case of the letters is important: Na (Sodium) is never NA or na or nA.
The mass of a nucleus depends on the total number of protons and neutrons that are part of it. The simplest way to talk about the mass of an atom is by using the atomic mass number. The atomic mass number, which has the symbol A, is just the total of protons and neutrons in the nucleus of a particular atom. It is really a number, not a measured mass. The atomic mass number of the atom shown in fig. 1 is 4. Atomic mass numbers are not shown on the Periodic Table of Elements. The exact mass of a particular atom is a number with a fractional part but the mass number is always a whole number. The exact mass always rounds off to the whole number found by counting protons and neutrons but they are not the same thing. Each element can have atoms with different masses (more on this later) and the mass shown on the table is an average of these.
To calculate the atomic mass number, just add up the number of protons and neutrons. A symbolic way to say this is: A = Z + n0. A is the atomic mass number, Z is the atomic number, or number of protons, and n0 stands for the number of neutrons.
The electrons never come near to the nucleus and instead orbit it at a great distance. The electron in a hydrogen atom has an orbit with a radius 60,000 times larger than the radius of the nucleus in a hydrogen atom. This makes it extremely difficult to draw a true-scale drawing of an atom. Neutral atoms have exactly the same number of electrons as they have protons. For this reason, the helium atom in fig. 1 has 2 electrons. We will consider all atoms to have the same number of electrons as protons for now. In reality atoms can gain or lose electrons fairly easily. Such changes in the number of electrons will be discussed elsewhere.
The elements were first discovered as elements during the 17th and 18th centuries. At that time scientists were making the first experiments that would break down everyday substances into the elements they are made up of. The things that these early chemists could not break down into simpler substances they called elements. This is the definition of ‘element’ that is important in chemistry: an element is a type of pure substance which cannot be broken down into simpler chemicals.
Atoms come in different varieties called elements. Elements are categories for atoms: they are not physical objects. Atoms can be different elements in the same way that candy comes in different flavors. Sour-apple-flavored candy you can eat; sour-apple flavor is a description, not something you can eat. In the same way, the element hydrogen is a description for a certain type of atom and is not itself an atom. The defining feature of an atom that makes it one element and not another is the number of protons in its nucleus, the atomic number (Z). Both the number of neutrons and the number of electrons can be different, but as long as the number of protons is the same then the atom is still the same element. Atoms of hydrogen all have 1 proton.
Atoms as found in nature already have a certain number of protons and neutrons. It is not possible to change these numbers. But we don’t need to change these numbers because nature has provided a wide variety of atoms with different numbers of protons and neutrons. To describe this variety, chemists use two terms: elements and isotopes. Elements are told apart based on the number of protons. Isotopes of an element all have the same number of protons but different numbers of neutrons. See fig. 2, below. In the figure two isotopes of hydrogen and two isotopes of helium are pictured. For simplicity, the electrons have been left out of the pictures. Both hydrogen atoms have one proton but one has no neutrons and the other has 1 neutron.
| Figure 2: Atomic Nuclei | |||
|
|
|
|
|
|
H Hydrogen-1
11H
1 p+ 0 n0 1 e– * |
H Hydrogen-2
21H
1 p+ 1 n0 1 e– * |
He Helium-3
32He
2 p+ 1 n0 2 e– * |
He Helium-4
42He
2 p+ 2 n0 2 e– * |
| *Electrons are not pictured in these diagrams of atomic nuclei. They are so small and so far away from the nucleus that, at the scale of these nuclei they would be about 390 m (or 1280 ft or ¼ mi) away and far too small to see. | |||
The symbol for an isotope shows both the atomic number (Z) and the atomic mass number (A). (See fig. 3, below). The symbol for the first isotope is 11H and it is called hydrogen-1. The name of an isotope includes both the name of the element and the atomic mass number (A) of the isotope. The symbol for the second isotope is 21H and it is called hydrogen-2. The symbol for the third isotope is 32He and it is called helium-3. The symbol for the fourth isotope is 42He and it is called helium-4. Almost all elements have two or more common isotopes, each with its own mass and number of neutrons. The atomic mass in the periodic table is an average of the masses of the different isotopes. So individual isotopes can be identified by their atomic mass number (A) which is just the sum of the number of protons and neutrons. An element on the other hand has a mass listed which is a weighted average of the masses of its common isotopes.
Isotopes of an element have nearly the same chemical and physical properties, they only differ in their atomic mass numbers. A complication for later discussion is that some isotopes are stable and some are radioactive. The radioactive isotopes decay to make isotopes of other elements but stable isotopes always retain their identity.
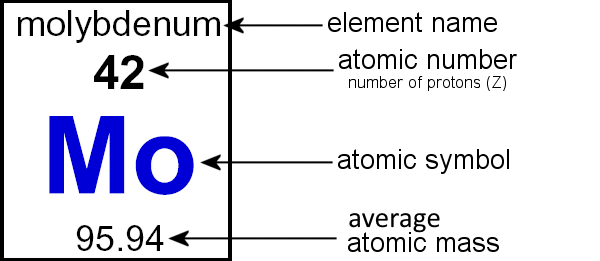
| Figure 3, An isotope of Molybdenum (Mo): Molybdenum-96 | |||
|
|
|
Z = 42 & A = 96
Z = the number of protons Z = the atomic number |
A = the atomic mass
A = the atomic mass number Z + n0 = A or A - Z = n0 |
Isotopes are variations on an element which have different numbers of neutrons. Another way that an atom can be modified while remaining the same type of element is for the atom to become an ion. Normally, we think of atoms having an equal number of protons and electrons. Such an atom is considered neutral because all of the positive charges (the protons) are neutralized by an equal number of negative charges (the electrons). The number of protons in an atom cannot be altered by normal everyday activities. However, electrons are so small that they can easily be added to or taken away from an atom. Ions are atoms which have gained or lost one or more electrons.
Imagine an atom of lithium, which has three protons. If it is neutral, then it also has three electrons. Picture it this way: (+ + +) for the protons and (–– –) for the electrons. With an equal number of each type of charge the overall charge is zero. If an electron is taken away from the atom then our symbols look like this: (+ + +) and (– –). Since there are more protons than electrons by one, the charge of the whole atom is +1. Positive ions are called cations and a cation forms when an atom loses one or more electrons. Atoms never gain or lose protons.
If we add an electron then our symbols look like this: (+ + +) and (– – – –). Since there are more electrons than protons by one, the charge of the whole atom is –1. Negative ions are called anions and an anion forms when an atom gains one or more electrons.
Since ions play a big part in understanding the chemical behavior of elements it is important to be able to calculate the charge of an ion. This can be done by making a table of plusses for protons and minuses for electrons but if there are a large number this can be inconvenient. To avoid having to draw a lot of plusses and minuses, it is simpler to use an equation. The charge of an atom equals the number of protons minus the number of electrons. Charge = p+ – e–.
Atomic symbols need to include the charge when an atom has become an ion. Charges are shown as a right superscript. See below for some examples of common ions including their charges. Mass number and atomic number have been left out for clarity.
Na+ Mg2+ Al3+ P3– S2– Cl–When a charge is +1 or –1 the symbol simply has a + or – without a number 1. For other charges the number comes first followed by the + or –.
Before the invention of the Periodic Table in the mid-1800s there was no way to organize all of the information chemists had gathered about the elements and the compounds that they form. Dmitri Mendeleev, a teacher of chemistry at a Russian university, wanted to be able to make the teaching of chemistry easier. In doing so, he invented the periodic table. He first made a version of the periodic table by putting elements and their properties on cards. The properties he looked at included things like melting point, boiling point, density, etc. The elements with similar attributes he placed in the same column. He also put them in order by atomic mass and found that a regular pattern resulted. Mendeleev had stumbled across the Periodic Law. The periodic law is the idea that the physical and chemical properties of the elements recur periodically if the elements are organized by the atomic number.
You will color your Periodic Table according to a color scheme shown by your teacher. The periodic table is divided up into groups and periods. The groups are columns and are numbered from 1 to 18. The elements in each group all have similar chemical and physical properties: this is what makes the periodic table a powerful tool. Take the Noble Gases, group 18. All of these elements share the common trait that they have practically no chemical reactivity at all. That means that they do not combine with other atoms to form compounds.
The periods are the rows of the periodic table. Across the periods the physical and chemical properties of the elements vary in a predictable way. The rows below the main table that start with lanthanum (La) and actinium (Ac) belong to periods six and seven. Notice that at element 56 (Barium, Ba) it goes straight to element 72 (Hafnium, Hf). Similarly, there is a gap in the numbering at element 88 (Radium, Ra). The missing elements (from 57 - 71 and from 89 - 103) are found below the main table because the table becomes inconveniently wide when you put them in their proper places.
Metals make up most of the elements in the periodic table. They are shiny, conduct electricity and heat well, and are easily bent and shaped. A sub-group of metals is called the Transition Metals and they are found in groups 3 - 12. Non-metals (groups 14 - 18) are dull-colored, do not conduct electricity or heat, and are brittle (or are gases). Semi-metals (the stair-step pattern) have traits of both metals and non-metals. The halogens (group 17) are very reactive elements. The noble gases (group 18) are very unreactive elements. The alkali metals (group 1) are very reactive metals and the alkali earth metals are similar, but less reactive metals. The rare earth metals (elements 57 - 70 and 89 - 102) are all quite similar to each other and are metallic in character. The rare earth metals can be split into two categories: the first row, starting with lanthanum (La), are called the Lanthanides and the second row, starting with Actinium (Ac) are called the Actinides. Hydrogen (H) falls into its own special group because it is not like any other element.
Use your notes and the information on your reading packet to answer the following questions. Answer using complete sentences. Also, use the questions as a way to help you to study the text: do not just look up ‘answers’ on the Internet.
| Name |
Symbol (AZX) |
Z
No. of p+ |
A - Z
No. of n0 |
A
Mass Number |
No. of e– |
| nitrogen-14 | 147N | 7 | 7 | 14 | 7 |
| nitrogen-15 | |||||
| oxygen-16 | |||||
| oxygen-17 | |||||
| oxygen-18 | |||||
| neon-20 | |||||
| neon-21 | |||||
| neon-22 | |||||
| potassium-39 | |||||
| potassium-40 | |||||
| potassium-41 | |||||
| calcium-42 | |||||
| calcium-46 |
| No. of Protons | No. of Electrons | Charge | Element Symbol with Charge | Cation or Anion? |
| 17 | 18 | Cl– | anion | |
| 3 | 2 | |||
| 3 | 4 | |||
| 8 | 10 | |||
| 11 | 10 | |||
| 26 | 23 |
| Name |
Symbol (AZXcharge) |
Z
No. of p+ |
A - Z
No. of n0 |
A
Mass Number |
No. of e– | Charge |
| nitrogen-14 | 147N3– | 7 | 7 | 14 | 10 | –3 |
| 157N+ | ||||||
| oxygen-16 | –2 | |||||
| 8 | 9 | 17 | +3 | |||
| 10 | 18 | 6 | +2 | |||
| 2010Ne3+ | ||||||
| 10 | 21 | 8 | ||||
| 10 | 12 | 5 | ||||
| potassium-39 | +1 | |||||
| 40 | 15 | +4 | ||||
| 4119K3+ |