In this lab you will investigate the power of gas pressure. In the school laboratory you will find a way to use atmospheric pressure to crush an aluminum drink can. At home you will investigate the power of microwaves to heat a gas to cause it to expand.

|

|

|
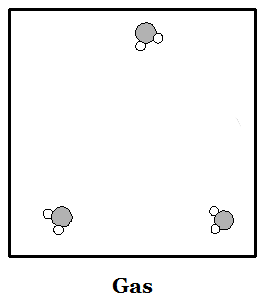
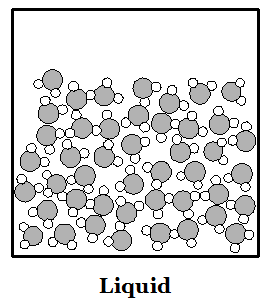
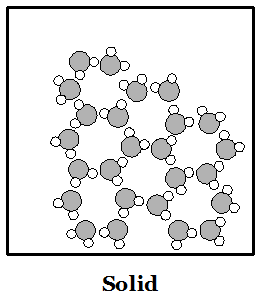
Everything in the world is made up of atoms and molecules. Both kinds of particles are subject to forces that attract them to other particles. They are also in constant motion--particles of matter never stop moving. If particles are moving very quickly then the attractive forces cannot hold them together and the material is in the physical state we call a gas. If particles slow down enough then the attractive forces can make them stick together. When particles stick together they can do so either as a liquid or a solid. Liquids have particles that are all touching, and stuck together, but in which the particles can all move around one another. Particles in a liquid move around each other like people at a very crowded party in a small house. Solids have particles that are all stuck together but the only motion they can make is to vibrate in place. In crystalline solids the particles may be arranged in a very regular pattern. In liquids the molecules are arranged randomly. The images at right can provide a basic idea of how to picture the phases of matter in the mind. The gas picture places the molecules much closer together than they are in reality.
When a substance we normally think of as a liquid is in the gas phase we call it a vapor. Water vapor is what makes humid weather feel sticky. Water vapor takes up enormously more volume than liquid water. For example, a 55-gallon oil drum completely full of water vapor will condense down to about one tablespoon of liquid water. Liquid water occupies about 0.07% of the volume of the same amount of water in the gas phase.
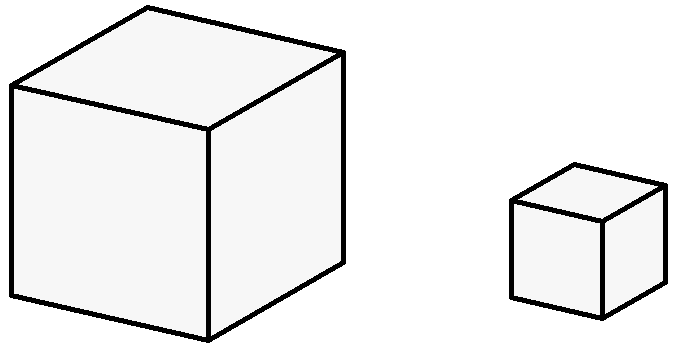
When scientists began to study gases very carefully in the
17th and 18th centuries certain simple proportions were
discovered. One of these, proposed by one Amadeo Avogadro,
is that there is a direct proportion between the amount of
gas and the volume of the gas that does not depend in any
way on the identity of the gas. In other words, the volume of a gas is directly proportional to the number of molecules, or the amount of gas. This sounds more
complicated than it is. Take a look at the illustration at
right. The cube on the left represents one unit of gas molecules in
one unit of volume. The cube on the right represents
 the volume of a gas which has 1/10 of the
amount of gas (or 1/10 of the number of molecules) in the bigger cube. It is, not by
coincidence, exactly 1/10 of the volume of the bigger cube.
So, when the number of molecules of gas is reduced by some factor, and
the gas pressure remains the same, the volume of the gas is
reduced by the same factor. If there is 1/2 the number of molecules of
gas then there is 1/2 the amount of volume.
the volume of a gas which has 1/10 of the
amount of gas (or 1/10 of the number of molecules) in the bigger cube. It is, not by
coincidence, exactly 1/10 of the volume of the bigger cube.
So, when the number of molecules of gas is reduced by some factor, and
the gas pressure remains the same, the volume of the gas is
reduced by the same factor. If there is 1/2 the number of molecules of
gas then there is 1/2 the amount of volume.
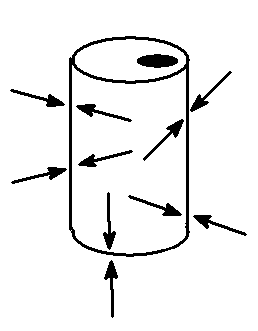
The atmosphere we depend on for our breath is a gas with a considerable amount of pressure. Every square inch of area of everything (at sea level) has a force equal to about 15 pounds on it. The reason we do not feel this pressure is because we and most of the objects around us have the same amount of pressure both inside and out. In the illustration at left of a drink can with an open top the arrows show the strength of the pressure at different points on the inside and the outside of the can. The pressure is the same everywhere and so the can remains unchanged by the force of that pressure. Some things do not have the same pressure inside and out but they must be sealed up tightly to prevent the pressure from becoming the same. Air in a car tire is pumped up to the equivalent of 3 atmosphere’s worth of pressure, if you include the pressure inside it before you started pumping. But once a tire is punctured it quickly loses the additional pressure and deflates. With less gas inside the volume of the tire decreases. Another example of a decrease in volume due to the loss of the gas inside is the rail tank car pictured below. One evening someone cleaned it with steam and then sealed up all the valves and spigots. The next morning imagine their surprise and dismay to see what atmospheric pressure could do to 7/8 inch thick steel!

Image source: http://thescienceclassroom.org/tank-car-crushed/ See also this video: https://youtu.be/Zz95_VvTxZM |
Another interesting law about gas behavior is called Charles’s Law and it is concerned with the proportion between the volume of a gas and its temperature. Earlier we discussed how particles act when they move more quickly or more slowly. A simple measurement that tells us something about the average speed of a collection of particles is the temperature. At low temperatures particles move slowly and at high temperatures they move faster. Faster moving gas particles move away from each other more quickly and so the volume of a gas has to be larger if the pressure of the gas is kept the same. At high temperatures the volume of a gas increases. At a lower temperature, slower moving gas particles move away from each other more slowly and so the volume of the gas is smaller for the same amount of gas at the same pressure. In other words, there is a direct proportion between temperature and volume of a gas. If temperature is decreased by a factor of two, then volume is decreased to one half its original size.
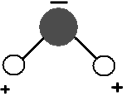
One way of making particles move faster that you may be familiar with is the microwave oven. Microwave ovens increase the temperature of the food inside them by emitting microwave radiation in a standing wave that causes particles with dipoles to spin around, bump into each other, and move faster. Molecules with dipoles include water and soap. Such molecules have a positive charge at one end and a negative charge at the other. See the illustration of a water molecule at right. Molecules without dipoles include the nitrogen and oxygen that make up the air we breathe. These kinds of molecules are not affected directly by the microwave radiation produced in a microwave oven. Instead they can only be heated up by being near a material made of polar molecules. An empty plastic container, which is made of non-polar molecules, will not heat up much in a microwave oven but it does get hot if there is water inside it absorbing microwave radiation.
The following list does not cover all possible hazards, just the ones that can be anticipated. Move slowly and carefully in the lab: haste and impatience have caused more than one accident.
Based on your reading of the background information devise a method to use atmospheric pressure to crush your aluminum can. Consider the materials listed in this lab handout as hints in making your plan. Discuss your plan with your lab partner but not with other groups: let everyone have a chance to figure it out. Write your plan clearly in your lab notebook in the Procedure section and show it to your teacher for approval before you begin.
You will need a bar of Ivory™ soap and a bar of some other brand and access to a microwave oven. Ivory™ soap is famous as the soap that floats. This is because it has thousands of tiny air bubbles in it. Most other soaps lack such air bubbles. See your teacher after class if you do not have a microwave at home so that you can arrange to do the experiment at school.
Answer all of the questions in a typed report. Use a numbered question and answer format.
In-class LabAnswer the questions above in a typed document on your own without working with another student.