

|
When allowed to form from a saturated salt solution, salt crystals take on a typical cubic shape. Crystal growth occurs on all faces about the same. At least, if the crystal is suspended in the middle of the solution. Growing on the bottom of a container can make the crystals slightly flattened as salt cannot reach the face that rests on the bottom. |
|
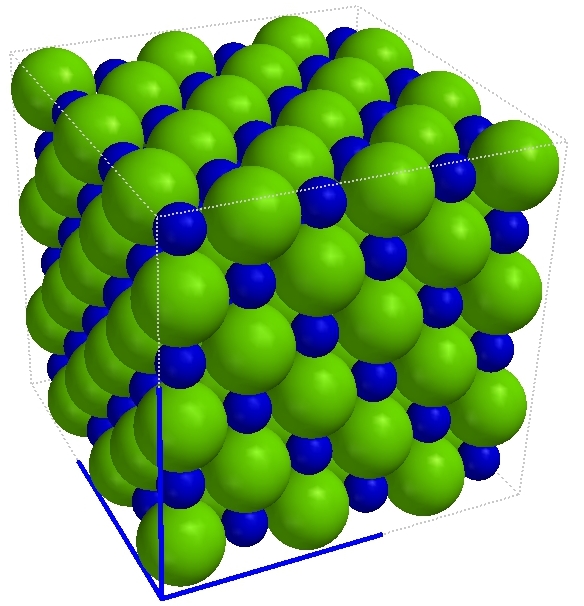
This is a view of a salt crystal at the atomic scale. Note that each sodium ion is surrounded by six chloride ions in the bulk of the crystal. This isn't true at the edges but in the interior each sodium ion has a chloride ion above, below, in front and behind, and to the left and to the right. |
Prussian Blue is a solid and in our lab it is suspended as tiny particles in water. When it reacts with the hydroxide ions produced by the ammonia solution, the following represents the formation of the brown precipitate (Fe(OH)3(s)).
The now freely dissolved [Fe(CN)6]4–(aq) ions are available to interact with salt crystals as they begin to form in the saturated salt solution.
|
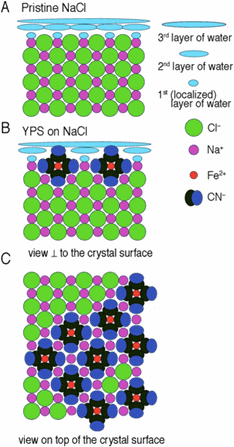
Figure 2 from reference 3 below. Models of the interaction of Prussian Blue on the NaCl surface as determined by Bode et al. (A) Side view of the blank NaCl crystal with three adsorbed water layers on the NaCl surface; (B) location of the [Fe(CN)6]4– ions sorbed onto the NaCl surface; (C) top view of the NaCl surface showing areas with no Prussian Blue, areas of densest possible [Fe(CN)6]4– ion coverage, and areas of a less dense coverage. Figure adapted from reference 1 below. The [Fe(CN)6]4– ions prevent normal crystal growth because they are the same shape as a group of sodium and chloride ions that make up a unit of the crystal, namely a sodium ion with six attached chloride ions. But this group of ions (a complex with the formula [NaCl6]5–) has a –5 charge while the [Fe(CN)6]4– ion only has a –4 charge. This difference requires one fewer sodium ion than usual but when it is left out the only attachment sites are negatively charged. A chloride ion cannot attach to such a location unless the [Fe(CN)6]4– ions detach from the surface, which they do not readily do. As a result the crystal cannot grow normally. |
|
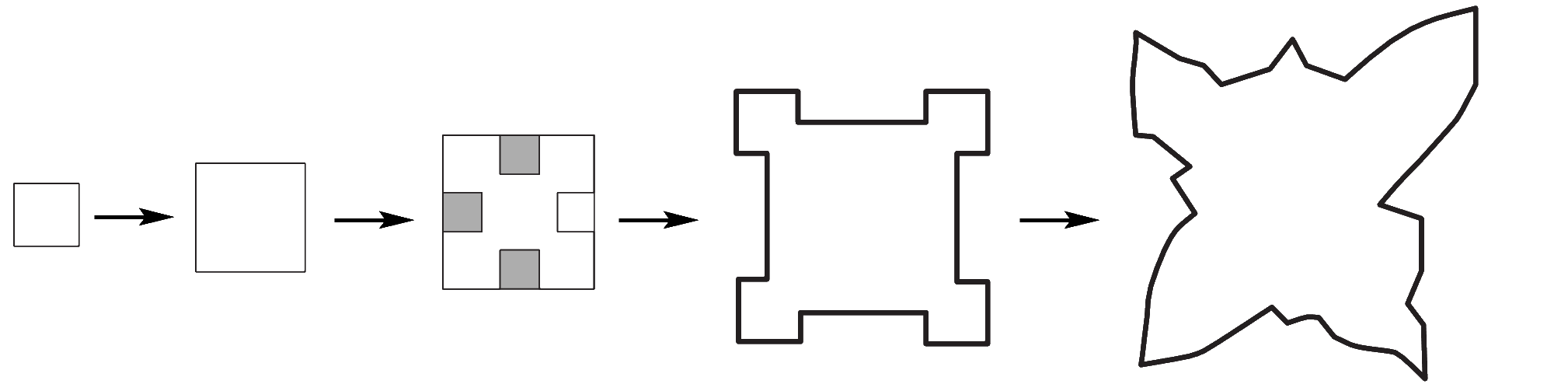
The [Fe(CN)6]4– ions cannot cover the entire surface of the salt crystals so growth continues but in a fashion that cannot lead to large, cubic crystals. In the image at left the process of crystal growth is depicted. First, crystals form normally. Then, shaded areas show where [Fe(CN)6]4– ions prevent further crystal growth. Sodium and chloride ions attach at the edges and on the corners and this leads to a branching growth pattern. (adapted from fig. 2 in reference 2) |