A weak acid is a weak electrolyte which dissolves in water mostly as molecules which retain their acidic proton. Only a small percentage of molecules are ionized to produce an anion (the conjugate base of the acid) and a hydrogen ion, or proton. When a weak acid reacts with a strong base it does so completely, even when the strong base is a limiting reactant. If there is not enough strong base to completely neutralize the weak acid then the solution becomes one in which the weak acid and its conjugate base both have non-zero initial concentrations. This situation is described as a buffer and the pH can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([A–]/[HA])provided the concentration is not so low as to be similar in size to the Ka of the acid. The pH of the solution can be calculated in this way up to but not including the equivalence point. The equivalence point of a titration is just when the number of moles of added base is stoichiometrically equivalent to the number of moles of weak acid in the original solution.
At the equivalence point, by definition, all of the weak acid molecules have been stripped of their acidic proton and exist in solution exclusively as the conjugate base:
HA + NaOH ⟶ NaA + H2OThe single arrow in the equation above shows that the reaction goes to completion and is not an equilibrium. The salt NaA is a strong electrolyte and dissociates completely in solution. The sodium ion is inert in acid-base reactions but the anion, A–, is a weak base. Since all of the sodium hydroxide has been used up and no HA remains the pH at the stoichiometric equivalence point is determined by this equilibrium:
A– + H2O ⇌ HA + OH–which is the hydrolysis of the weak base that is the conjugate base of the original weak acid.
After the equivalence point there is no more HA and the added sodium hydroxide, being a much stronger base than A–, dominates the solution in terms of determining the pH. To calculate pH the moles of excess sodium hydroxide divided by the total volume gives the concentration of OH–.
A weak base is a weak electrolyte which dissolves in water mostly as molecules which do not bind to an acidic proton. Only a small percentage of molecules are ionized to produce the conjugate acid of the base and a hydroxide ion. When a weak base reacts with a strong acid it does so completely, even when the strong acid is a limiting reactant. If there is not enough strong acid to completely neutralize the weak base then the solution becomes one in which the weak base and its conjugate acid both have non-zero initial concentrations. This situation is described as a buffer and the pH can be calculated using the Henderson-Hasselbalch equation provided the concentration is not so low as to be similar in size to the Ka of the acid form of the base. The A– in the expression is the weak base being titrated and the HA is its conjugate acid. The pKa is that of the conjugate acid. The pH of the solution can be calculated in this way up to but not including the equivalence point.
At the equivalence point, by definition, all of the weak base molecules have bonded to an acidic proton and exist in solution exclusively as the conjugate acid:
A– + HCl ⟶ HA + Cl–The single arrow in the equation above shows that the reaction goes to completion and is not an equilibrium. The chloride ion is inert in acid-base reactions but the acid, HA, is a weak acid. Since all of the hydrochloric acid has been used up and no A– remains the pH at the stoichiometric equivalence point is determined by this equilibrium:
HA ⇌ H+ + A–which is the hydrolysis of the weak acid that is the conjugate acid of the original weak base.
After the equivalence point there is no more A– and the added hydrochloric acid, being a much stronger acid than HA, dominates the solution in terms of determining the pH. To calculate pH the moles of excess hydrochloric acid divided by the total volume gives the concentration of H+.
An indicator is a weak acid which has different visible colors depending on whether it is protonated or not. When the indicator is in sufficiently acidic solution it has the color of the form which has an attached hydrogen ion. When the indicator is in sufficiently basic solution it has the color of the form which has no attached hydrogen ion.
The color-change range of an indicator spans about 2 pH units. This has to do with the ratio of the concentration of the protononated form (HIn) to the concentration of the de-protonated form (In–). If the concentration of HIn is ten or more times greater than the concentration of In– then the color of HIn dominates. If the concentrations of HIn and In– are close to being the same, that is, close to having a one-to-one In–/HIn ratio, then the color observed is a mixture of the two forms’ colors. If the concentration of In– is ten or more times greater than the concentration of HIn then the color of In– dominates.
Figuring out which form is in the majority is made simple by the use of the Henderson-Hasselbalch equation:
The pH in the solution in a titration is determined completely by the composition of the solution and is not really affected by the small amount of indicator present. In this equation the value of the pH can be substituted in and the ratio [A–]/[HA] can be understood to be [In–]/[HIn]. All that is needed is the pKa of the indicator, which in the case of bromothymol blue is 6.8. The pKa of phenolphthalein is 9.2. The pKa of methyl red is 5.4.
It is necessary to know the theoretical pH at the equivalence point of a titration in order to select the correct indicator. The indicator cannot show the equivalence point precisely because it changes color over a range of 2 pH units. However, it can show the endpoint of the titration. The rate of change of pH just before and after the equivalence point is practically infinite and the range of pH from just before to just after equivalence can be 4 or 5 pH units. As long as the indicator changes color within this larger range it will clearly show the end of the titration, that is when the volume delivered up to that point contains a stoichiometrically equivalent amount of acid or base.
Depending on the amount of titrant added there are four different methods needed to calculate the pH of the solution for that amount of added strong acid or strong base.
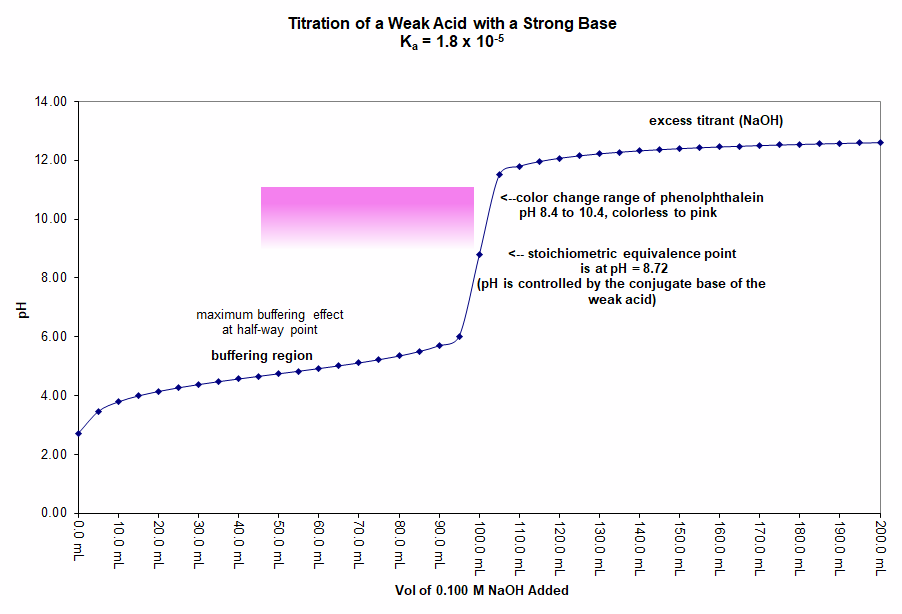
In this graph 50.0 mL of a 0.20 M solution of acetic acid (CH3COOH) is titrated with a 0.10 M solution of sodium hydroxide (NaOH). The appropriate indicator for the titration is phenolphthalein because it has a color-change range from pH 8.4 to 10.4. The pH expected at the equivalence point is 8.72 and the pH jumps from 6.7 at one mL of base before equivalence to 10.8 at one mL of base past equivalence.
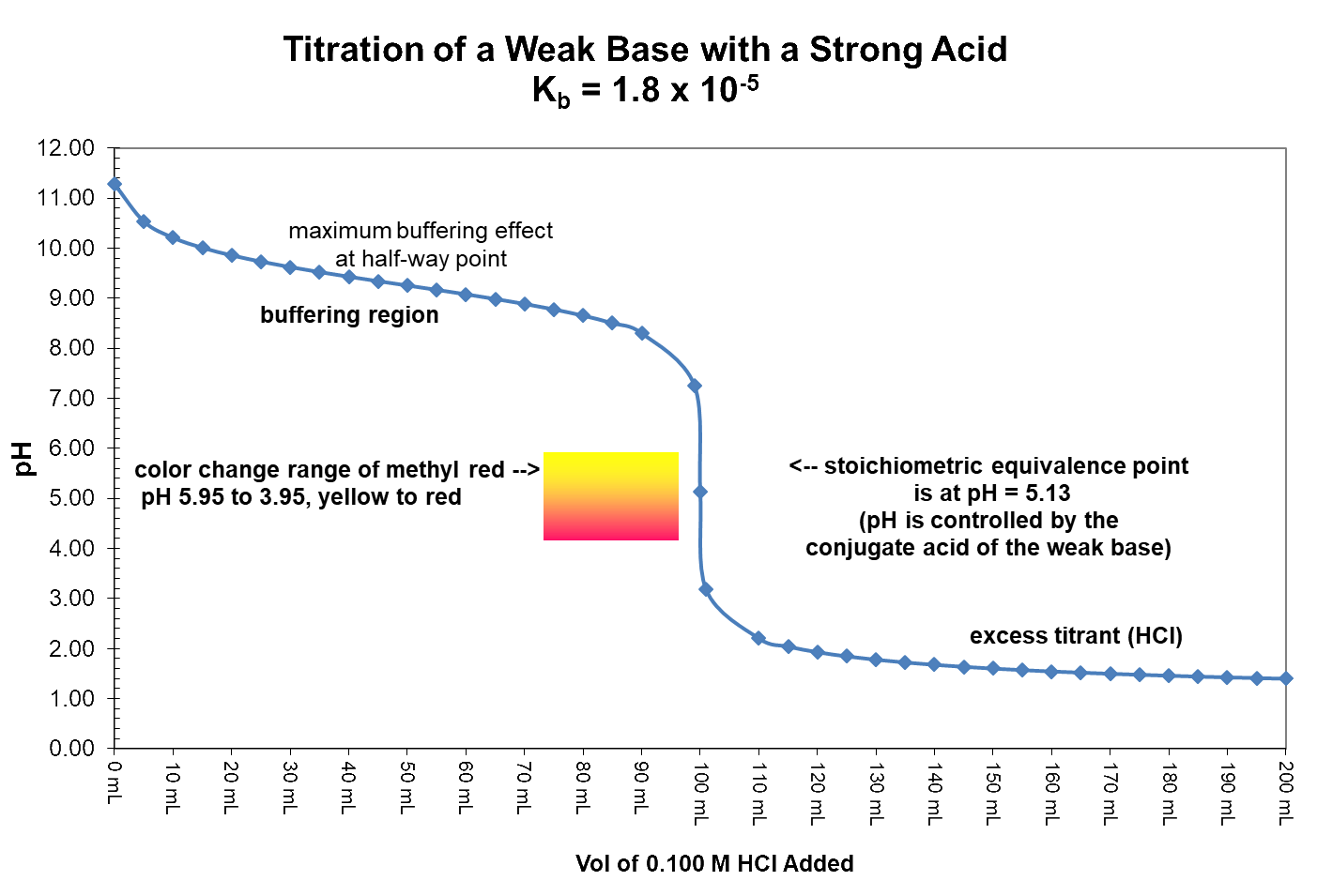
In this graph 50.0 mL of a 0.20 M solution of ammonia (NH3) is titrated with a 0.10 M solution of hydrochloric acid (HCl). The appropriate indicator for the titration is methyl red because it has a color-change range from pH 5.95 to 3.95. The pH expected at the equivalence point is 5.13 and the pH jumps from 7.2 at one mL of acid before equivalence to 2.2 at one mL of acid past equivalence. The pH at equivalence is below 7 because the species in solution that controls pH at that exact point is the conjugate acid of the weak base being titrated.
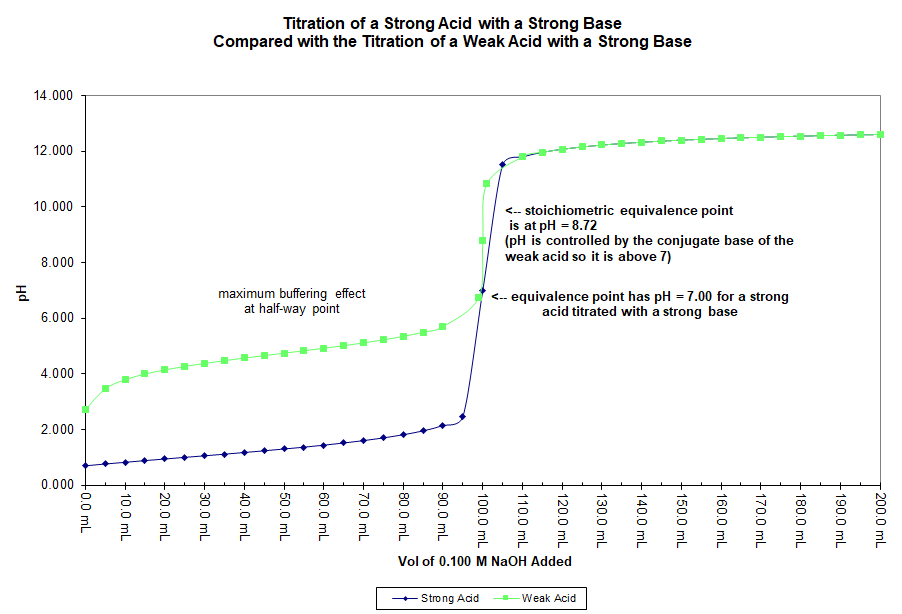
In this graph 50.0 mL of a 0.20 M solution of acetic acid (CH3COOH) is titrated with a 0.10 M solution of sodium hydroxide (NaOH). For purposes of comparison, a similar titration of 50.0 mL of a 0.20 M solution of hydrochloric acid (HCl) is also displayed on the same graph.
While the strong acid has a pH at equivalence of 7.00 the weak acid, in this case, has a pH of 8.72. This is because the chemical species present at equivalence in each case are different. For the strong acid/base titration the species present at equivalence have no effect on pH. For example, the chloride ion (Cl–) and the sodium ion (Na+) are not basic and not acidic.
At equivalence for the weak acid titration the species in solution include the sodium ion, the conjugate base of the acid, and water. The weak base is the only one of these that has an effect on pH and since it is a base, the pH is above 7 at equivalence.