
Your teacher will show you a demonstration in which water ice is placed on two different surfaces.
|
Molecule: a tiny particle of matter made of two
or more atoms. All molecules are a little bit sticky.
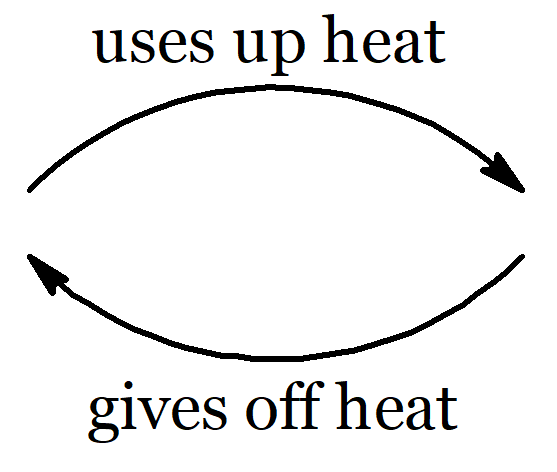
All molecules are in constant random motion. Heat: the energy that makes molecules move faster if you add it to the molecules. Molecules move slower when they give up heat to something else. Heat is the power to change temperature or to cause a phase change. Temperature: a measure of the speed of molecules. Higher temperature means faster molecules. |
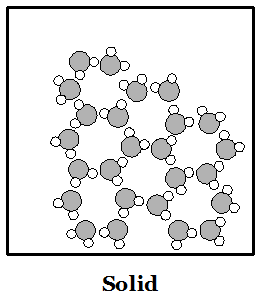
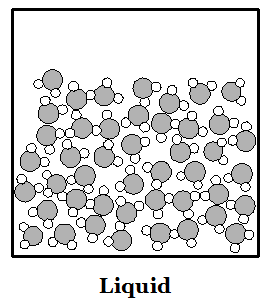
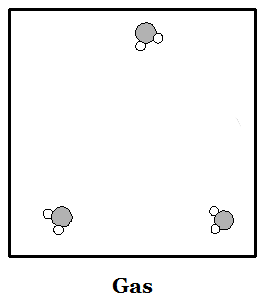
Phases of Matter: Solid: a phase matter
in which the molecules are moving so slowly that their
stickiness makes them stay in place. Molecules sit in
one place like children sitting at their desks. Liquid: a phase of matter in which molecules move a bit faster but are all still stuck together. Molecules move around a bit like children standing in a clump waiting to get into a classroom. Gas: a phase of matter in which molecules move so fast that their stickiness is not strong enough to keep them together. Molecules move around like children on the playground, bouncing off of each other and things with lots of empty space in between. |

|

|

|

|

|
Water (H2O) molecules are made of two atoms of hydrogen bonded to an oxygen atom. Hydrogen atoms are smaller than oxygen atoms. In the pictures above you can see how water molecules are arranged in each phase. Ice floats because of all the empty spaces between water molecules when water is solid. When a solid melts or a liquid evaporates, heat is used up. When a gas condenses or a liquid freezes, heat is given off.
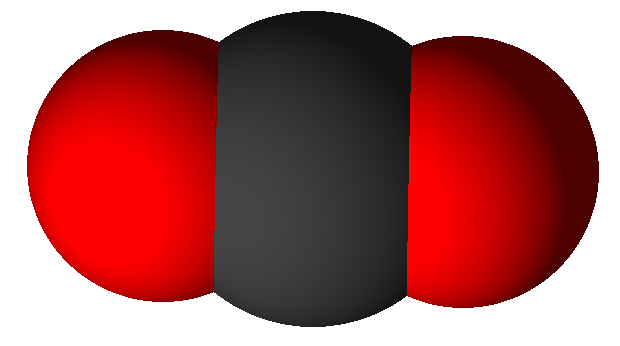
In the spaces below create some molecule drawings for the phases of carbon dioxide (CO2). A molecule of carbon dioxide has one atom of carbon in the middle bonded to two atoms of oxygen and looks like this:
|
(tightly packed, nice and regular) Solid |
(a bit looser but random) Liquid |
(very few and far apart) Gas |
Dry ice is solid carbon dioxide (CO2). It has a temperature of –78°C or –108°F. As it absorbs heat it turns directly into a gas, a process called sublimation. To be safe with it, follow the rules below:
Your teacher will show you how to do each of the demonstrations below. You will have a chance to try each of them and record your observations.
Singing SpoonHold a piece of dry ice with a pair of tongs pressed against the table top. Press a warm spoon against the dry ice.
|
Air HockeyUse tongs to place a piece of dry ice on the table. Let it sit awhile: look at it closely while you wait about a minute. Once the bottom of the piece of dry ice has flattened out, try hitting it from the side to make it slide.
|
Pop-top Film CanistersUse tongs to place a small piece of dry ice in one of the film canisters. Seal it up (this is safe because the top will pop off before the container builds up enough pressure to explode).
|
FogUse tongs to place one or two pieces of dry ice into a cup of cool water. Use tongs to place one or two pieces into a cup of very warm water.
|