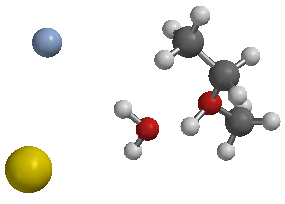
All of the objects in this image are particles
The two on the right are molecules
The two on the left are atoms

|
|
Figure 1 All of the objects in this image are particles The two on the right are molecules The two on the left are atoms |
Chemistry is the study of matter and its transformations. To begin to understand chemistry it is necessary to have an idea of the basic components of matter. Matter is made of particles. Every smooth surface and every fluidly pouring liquid is made up of separate little bits of matter. A cascade of water has more in common with pouring out a bucket of ball bearings than it at first appears. Water is made of molecules, which are a kind of particle. Particles are objects made of at least one atom. A particle may also be made of two or more atoms bound strongly together by a chemical bond. When atoms are bound in this way the do not easily separate again and this type of particle is called a molecule. A molecule is a particle of matter made up of two or more atoms that are bound together so that the whole thing has a specific structure and moves and acts as a single unit. All materials are made of particles. For some materials, the smallest particles are atoms, and for others the smallest particles are molecules. An atom is the smallest kind of particle and they can be classified into different types. The types of atoms are called elements and the usual reference for all the types of atoms that there are is called the periodic table. The table has space for 118 elements but only about 92 occur naturally on the Earth.

|
|
Figure 2 A sample of gaseous oxygen (O2) |
|
|
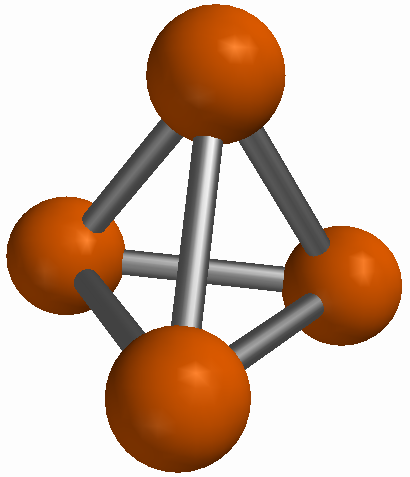
Figure 3 A molecule of phosphorus (P4) |
An elemental substance is a substance that is made of only one type of atom. The definition of the word element is that it is a category or a description for a particular kind of atom. A single atom can only be one type or element. But a single atom is not actually a substance: it is just the smallest particle of an actual material. Samples of elemental substances are materials which consist of many atoms but only one type of atom. The atoms themselves can be arranged in a number of different ways. Some elemental substances are monatomic: they consist of particles which are only one atom in size. Elemental substances of this kind are usually found as gases. Some elemental substances are diatomic molecules, which are molecules made of only two atoms. Diatomic molecules of elemental substances have two of the same type of atom chemically bound together. Examples include hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2). Elemental substances of this kind may be solids, liquids, or gases. Still other elemental substances make bigger molecules. Examples include phosphorus (P4) and sulfur (S8). Finally, some elemental substances do not form molecules but instead create a sort of network for themselves where all the atoms are bound together in what is effectively one gigantic molecule. Both graphite and diamonds are made only of carbon atoms: they differ because of differences in the way the atoms are networked together.
|
|
|
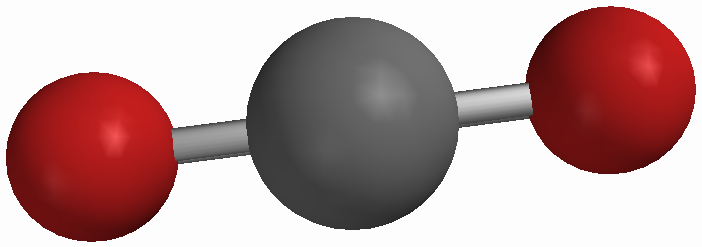
Figure 4 At left: A single molecule of carbon dioxide (CO2) At right: Solid CO2 |
|
A compound is a substance made up of two or more types of atoms which are chemically bound together in a specific ratio and with a specific structure. Compounds come in several different basic varieties. One of these is a molecular compound. The smallest particles of such compounds are molecules and a sample of a compound is made up of many, many copies of the same molecule. Some examples include water (H2O), carbon dioxide (CO2), and methane (CH4). Some molecular compounds are diatomic, that is their molecules have just two atoms. Examples include carbon monoxide (CO), hydrogen chloride (HCl), nitrogen monoxide (NO), and bromine monofluoride (BrF). Like many forms of matter, molecular compounds can exist as solids, liquids or gases. In all of these phases the molecules of the compound remain intact: no chemical bonds between atoms break when a liquid boils or a solid melts.
|
|
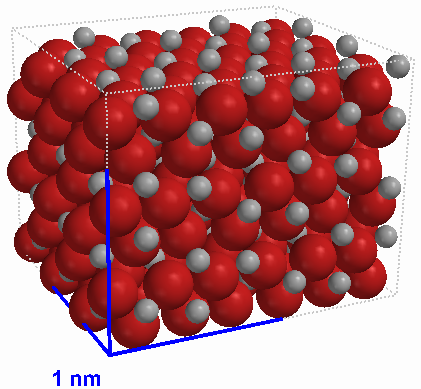
Figure 5 A small crystal of iron(III) oxide (Fe2O3) The large atoms are oxygen, the smaller atoms are iron |
Another basic variety of compound is the ionic compound. An ionic compound has a specific ratio of atoms of different types but is not made of molecules. Instead, a sample of an ionic compound is made up of at least two basic particles: positive ions and negative ions. These ions have strong electrical charges, which hold the ions together in the solid form in a rigid crystal lattice. When ionic compounds melt they lose their rigid structrure and the separate positive and negative ions move independently. Similarly, when ionic compounds dissolve in water their ions separate. One example of an ionic compounds is table salt, also known as sodium chloride (NaCl). Other examples include sodium fluoride (NaF), which is found in toothpaste, and iron(III) oxide (Fe2O3), also known as rust.
So far these notes have described pure substances, or materials that have a specific chemical composition that is the same throughout the material. In chemistry purity is a matter of whether one substance is mixed with another or not. If there is more than one elemental substance or compound present then the material is a mixture. Mixtures are different from chemical compounds because they can have a variable composition. In other words, the different pure substances in the mixture are not necessarily fixed at one specific ratio. Water is a compound of hydrogen and oxygen because in all molecules of that pure substance there are exactly two atoms of hydrogen and one atom of oxygen (H2O). If hydrogen gas (H2) is mixed with oxygen gas (O2) they can be mixed in any proportion from 1% hydrogen/99% oxygen to 99% hydrogen/1% oxygen or anything in between.
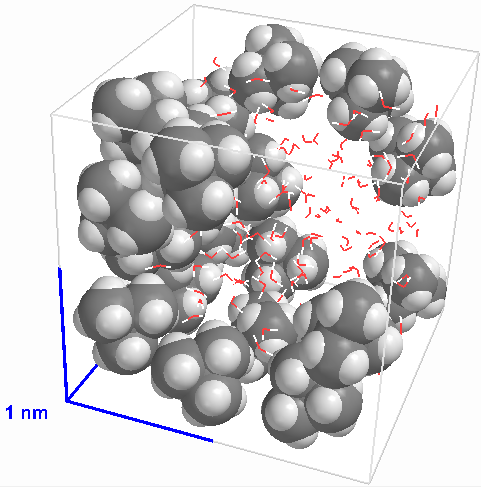
|
|
Figure 6 A sample of air, a homogeneous mixture |
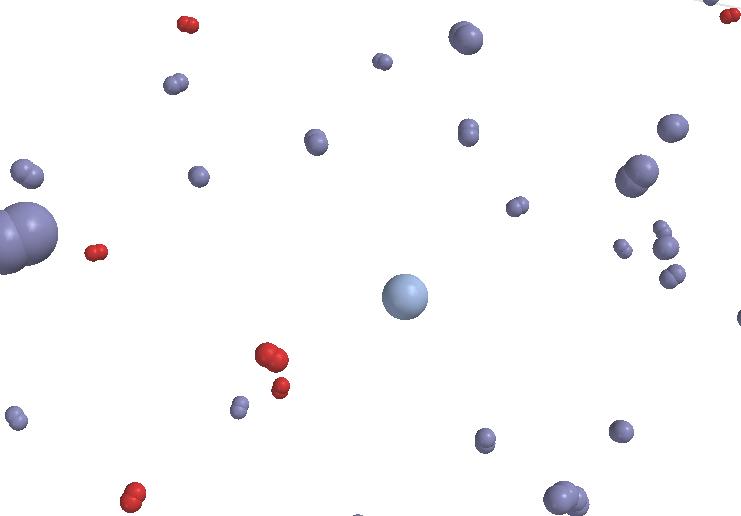
|
|
Figure 7 A heterogeneous mixture of an oily molecule with water (the water molecules are shown only in outline) |
There are two different kinds of mixtures. A homogeneous mixture is one in which the components of the mixture are mixed completely evenly with no clumps. One example of a homogeneous mixtures is the atmosphere of Earth, which is 78% nitrogen (N2), 21% oxygen (O2) and 1% argon (Ar). Another example is salt water which can range in composition from a teaspoon of salt in an olympic-sized pool, to the ocean, a solution that is 3.5% salt by mass. Alloys are mixtures of metals. When zinc and copper and melted together the combination is called brass. Tin and copper make bronze. The color and other properties of these alloys change with different proportions of each metal.
Carbonated soda in a can is a homogeneous mixture of water, sugar, carbon dioxide, and other ingredients. But when the can is open and bubbles of carbon dioxide begin to form and bubble up to the top the mixture becomes heterogeneous. A heterogeneous mixture is one in which the components of the mixture are not mixed entirely evenly. There are clumps of one material embedded in another. The bubbles in an effervescent glass of soda are a separate substance from the rest of the beverage and there is no liquid inside them. Another example of a heterogeneous mixture is beach sand. Sand is a complicated mixture of a variety of different minerals, each with their own chemical composition, along with bits of shells and other organic debris. River mud in a glass of water, no matter how strongly it is stirred, will always remain a separate material from the water. The pieces may be very small but the silt that makes up the mud never truly dissolves in the water. This is proved by the fact that eventually it all settles out to the bottom of the glass. Shaving foam is a heterogeneous mixture of a gas and the water and other molecules that make up the walls of the bubbles. Oil and vinegar salad dressing is a heterogeneous mixture, too, with clear layers showing the separate components of the mixture.
Molecular-scale models of mixtures always make clear whether a mixture is homogeneous or heterogeneous. Actual mixtures may be hard to distinguish in practice. This is because some heterogeneous mixtures look like homogeneous mixtures. For example, a creamy salad dressing has no visible layers of separate oil and vinegar. This is because another component of the mixture has allowed the molecules of oil and water to mix much more thoroughly. Any material that allows two otherwise un-mixable components to mix together is called an emulsifying agent. Ice cream is another example of a heterogeneous mixture that looks homogeneous.
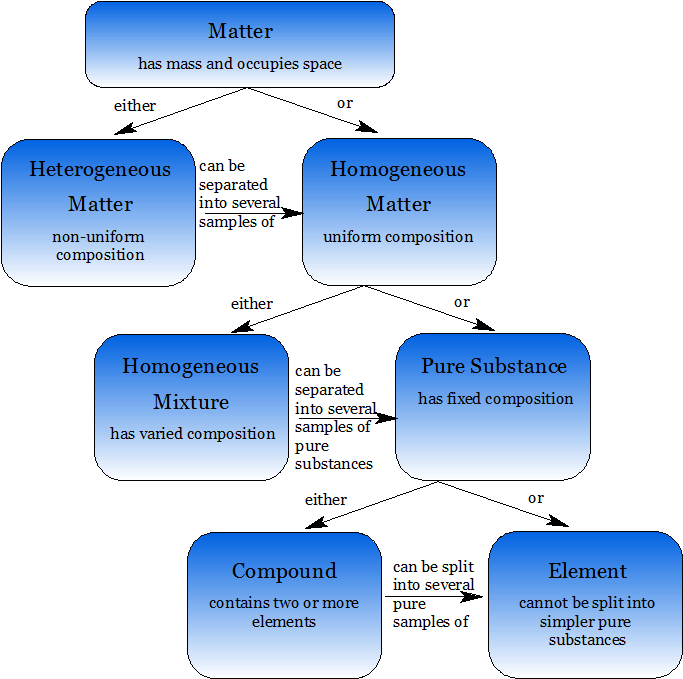
Here is a summary of this discussion of the definitions of the various kinds of matter:
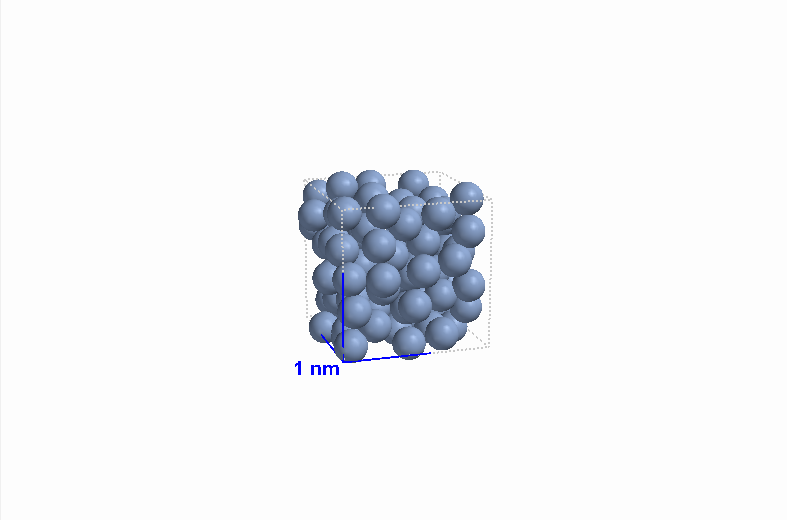
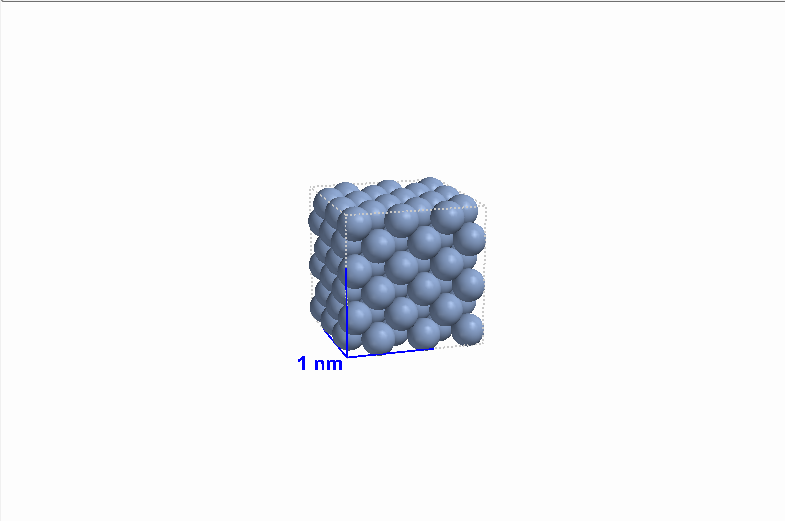
The phases of matter are solid, liquid and gas. A solid is when a material’s particles are very close together. Often they are arranged a symmetrical pattern, called a crystal. Some solids, for example glass, do not have regular repeating and symmetrical patterns of atoms or molecules. Solids cannot be compressed because there is very little space between the particles for the atoms to move into. On average, particles in a solid are stationary but from moment to moment they do jiggle around without ever really moving from their original position. Solids have a specific volume and shape which do not depend on their circumstances. A liquid is when a material’s particles are very close together but not bound so strongly that they cannot move from place to place. Liquids also cannot be compressed due to the lack of spaces between particles. When compressed with enough pressure, however, liquids can be forced to crystallize at temperatures above their normal melting points. On average, particles in a liquid do in fact move from place to place and around one another. This is a slow process called diffusion. The particles in a liquid are so close together that they cannot move past one another quickly. Liquids assume the shape of their containers because they do not have a structure that gives them a three-dimensional shape.
In both solids and liquids forces of attraction between particles keep the particles from separating from one another. You can think of these weak forces as a kind of molecular stickiness. These forces are weaker than chemical bonds that exist in molecules. The amount of attractive force (stickiness) is the same at all times and depends only on how far apart the molecules are. If molecules are made to speed up by adding heat they can break the weak bonds to undergo a phase change. A phase change is when a material changes from one of the phases of matter into another. For example, a liquid can be heated enough that it will evaporate and turn into a gas. Or it could be cooled enough to solidify. Added heat causes particles to increase the average speed of their motions and when they move faster they can more easily overcome the attractive forces holding them together. When heat it removed, particles move more slowly and the attractive forces are then strong enough to make them stick together. Adding heat makes a solid turn into a liquid and then a gas. Taking heat away turns a gas into a liquid and then a solid. There is no such thing as ‘cold’--when something has a low temperature it means that heat has been taken out of it.
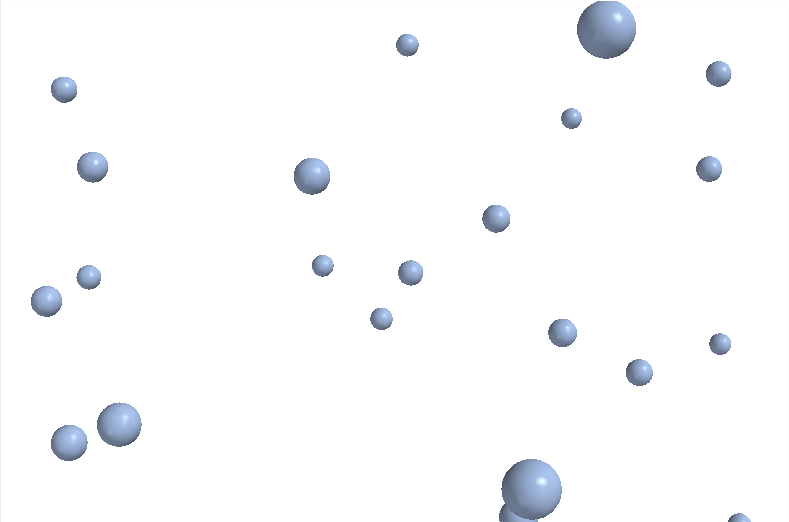
A gas is when the particles of the material are very far from one another. The forces of attraction that hold particles together in the solid or liquid phase have little opportunity to act in a gas because a gas is mostly empty space. In fact, gases form from liquids when they are heated strongly enough so that all of the particles have enough energy to break the forces of attraction that would keep them in the liquid phase. The particles of a gas can move for long distances without hitting another particle or the walls of a container. Gases assume both the shape and the volume of their container. Because they are really almost entirely empty, gases are very compressible. By reducing the volume of a gas the particles simply have less room in which to move. When compressed the particles themselves always remain exactly the same size: only the volume in which they are allowed to move is changed.
 |
 |
 |
| Figure 8 Gaseous argon (Ar) |
Figure 8 Liquid argon (Ar) |
Figure 8 Solid argon (Ar) |