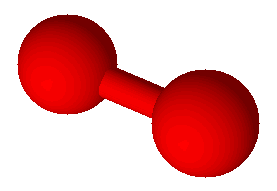
Oxygen (O2)
Hydrogen
Chloride (HCl)
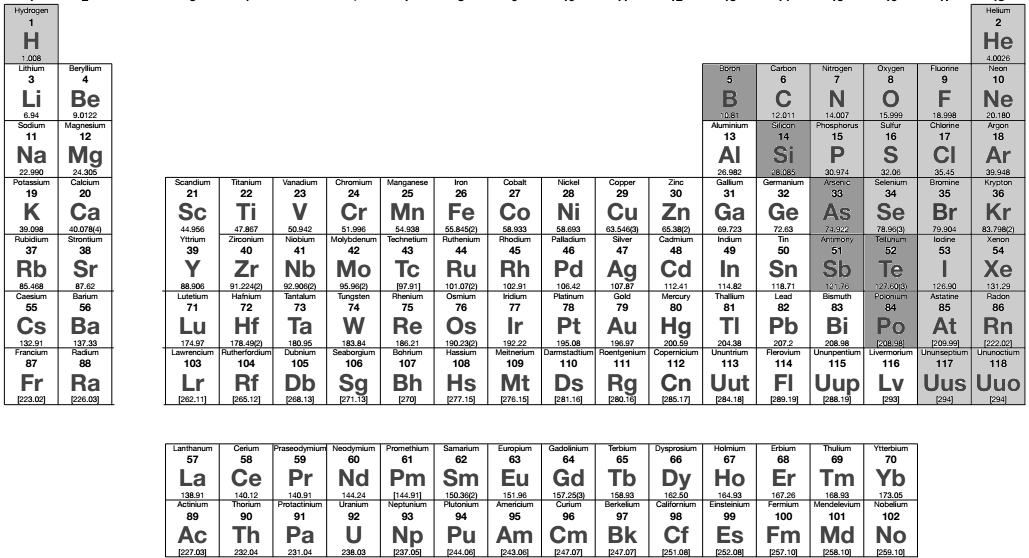
The periodic table of elements shows basic information about all the different types of atoms that make up all of ordinary matter. There is a basic division in the table between elements considered to be metals and elements considered to be non-metals. Metals make up most of the elements in the periodic table. In the graphic at right the metals are white. The non-metals are light-grey. A few elements fall in between these categories and are called semi-metals. These are colored dark gray. Familiar metals include iron, silver, gold, copper, and zinc. Usually gray or silver in color, metals are good conductors of heat and electricity and most are not brittle. Metals deform instead of breaking when struck hard with a hammer. Only one metal, mercury (Hg), is a liquid at room temperature. Familiar non-metals include carbon (charcoal), sulfur, oxygen, and neon. Non-metals that are solids tend to be brittle and are usually poor conductors of heat and electricity. Many pure non-metals are gases and one, bromine (Br2), is a liquid. For a material to be called an element it must be made of only one kind of atom.

Oxygen (O2) |

Hydrogen Chloride (HCl) |
Atoms can bond together in a number of different ways. As elements, non-metal atoms can bond together to form molecules , which are collections of atoms that move as a group. Metallic elements bond together with a special type of bond un-creatively called metallic bonds. These bonds form vast networks that allow electrons to move from atom to atom like boats in an ocean dotted with islands. Compounds are formed when atoms of different elements bond to one another. When non-metals bonds to other non-metals they do so by sharing electrons. The electrons in the bond spend most of their time between the nuclei. Being negatively charged, electrons attract both atomic nuclei, which are positively charged. Because the electrons that create the bond come from the valence shell of the atoms this type of bond is called a covalent bond .
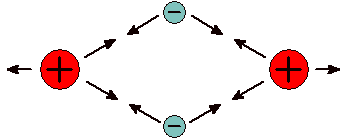
Covalent Bond Large atomic nuclei bond due to attractions to tiny electrons. |
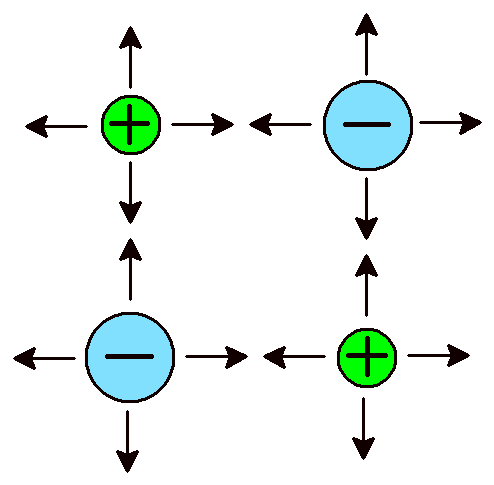
Ionic Bonds A network of attractions between ions. |
Covalent bonds hold atoms together because shared electrons attract both atoms. When atoms are held together by covalent bonds they form molecules. Oxygen is an example of an element which naturally forms molecules; in this case with two atoms each: O2. Oxygen is a diatomic molecule, one with just two atoms. There are seven elements, including oxygen, that exist naturally in their pure form as diatomic molecules. Commit these to memory: hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2). There are compounds with diatomic molecules, too, such as HCl. Hydrogen chloride has one atom of hydrogen and one atom of chlorine and they are bound together by a covalent bond.
Molecules of compounds are collections of atoms covalently bound together and which move as a unit. Molecules themselves can bond to one another by intermolecular bonds. This type of bonding is what is responsible for the ability of molecular compounds to become liquids or solids. The molecules stick to one another in solids and liquids. When they evaporate the molecules remain whole but the bonds between them are broken. Intermolecular bonding will not be a focus of the current activity.
Another type of bond is at the opposite end of the chemical bond spectrum, the ionic bond . Ionic bonds form between metal atoms and non-metal atoms and come about because positively and negatively charged atoms attract due to their opposite charges. The atoms themselves have either gained or lost one or more electrons. When atoms lose electrons they become positively charged. Metals characteristically form positively charged ions. Chemists refer to positive ions by the name cation . When atoms gain electrons they become negatively charged. Non-metals form negative ions, also called anions . Ionic bonds refer to strong attractions between ions of opposite charge. Ionic bonds do not allow atoms to form molecules. Instead, the atoms create a network of alternating cations and anions. Sodium chloride (NaCl) is an excellent example of a familiar ionic compound. Its atomic structure consists of repeating sodium (Na+) and chloride (Cl–) ions. A single crystal of granulated salt might contain 1 × 1018 cations and 1 × 1018 anions (that’s a 1 followed by 18 zeroes). Regardless of the total number, there is always one cation for every anion.
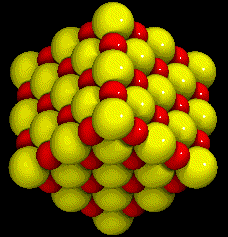
|
| NaCl Crystal |
Ionic compounds are combinations of atoms of different elements that are bound together by ionic bonds. They are always a combination of at least one metal with at least one non-metal. The formulas of ionic compounds do not show the number of atoms of each element found in a molecule of the compound. Ionic compounds do not form molecules. Instead, the formula of an ionic compound shows the lowest whole-number ratio of ions in the compound. In sodium chloride (NaCl) there is one sodium ion for every chloride ion. In calcium chloride (CaCl2) there is one calcium ion for every two chloride ions. Ionic compounds are characterized by having very high melting points and boiling points. Sodium chloride, for example, melts at 801°C and boils at 1413°C. Ionic compounds are strong electrolytes, poor conductors of electricity as solids, and excellent conductors as liquids. Crystals of ionic compounds vary in the details of their structure but mainly they consist of alternating positive and negative ions, as in the image of NaCl at right. The sodium atoms in the crystal are smaller and darker.
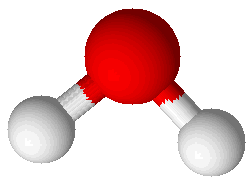
Water (H2O) |
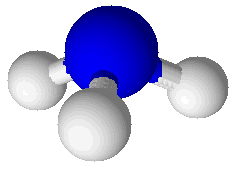
Ammonia (NH3) |
Molecular compounds are combinations of atoms bound together by covalent bonds to make molecules. Molecules are groups of atoms bound together in a particular pattern with a particular number of each kind of atom. For example, molecules of water (H2O) have a central oxygen atom bound to two hydrogen atoms. A picture showing a water molecule is displayed at right. Bonds in this picture are shown as cylinders that connect the spheres representing atoms. The oxygen atom is larger than the two hydrogen atoms. Another molecular compound is ammonia (NH3), which has one nitrogen atom and three hydrogen atoms. It is shown at right, below the water molecule. Molecular compounds are characterized by having relatively low melting points and boiling points. Ammonia melts at –78°C and boils at –33°C. Ammonia is a gas in its pure state at room temperature. Water melts at 0°C and boils at 100°C: this common, familiar compound is actually very unusual in that its melting and boiling points are so high for such a small molecule. Molecular compounds are either non-electrolytes or weak electrolytes. They are poor conductors of heat and most do not conduct electricity. When molecular compounds solidify as crystals they do so as a result of intermolecular bonds. This type of attraction between molecules is much weaker than either covalent bonds or ionic bonds. One way to compare the strength of intermolecular bonds to covalent
Binary molecular compounds are compounds made of two elements. Some examples are water (H2O), ammonia (NH3), carbon dioxide (CO2), and methane (CH4). Of these four examples, only one is usually know by its formal, systematic name. The International Union of Pure and Applied Chemistry (IUPAC) is the organization which publishes the conventions used to name chemical compounds. For this reason chemists all over the world use the same names for the same compounds. Having a system to determine the single, unique name for a compound is very important because without such a system confusion could result. If different chemicals had the same or nearly the same name it would lead to mistakes in which the wrong chemical is added to a reaction.
Naming binary molecular compounds is quite simple. More complicated compounds are much more difficult to name but will not be covered in this activity. The name of a compound gives both the names of the elements that compose it and the number of atoms of each element found in the compound. This is accomplished by using prefixes that indicate the number. The prefixes are shown in a box above.
The first step in naming a compound given its formula is to recognize whether or not it is molecular or ionic. Molecular compounds are those made of non-metals. Compound formulas containing a metal atom are usually ionic and follow a different system for naming them. Once a compound is identified as molecular prefixes are assigned to each element’s name and the second element’s suffix is changed to –ide. The mono– prefix is never used for the first element in the name of a molecular compound. Here are some compounds along with their names:
Notice in the names at right that the final “a” or “o” of the
The electrons in atoms are organized into different levels. Only the outermost level of electrons in an atom are involved in forming bonds to other atoms. This outermost shell of electrons is called the valence shell. The inner electrons are called core electrons. The number of valence electrons is an important factor in determining how an element will react with other elements, that is, in how it forms compounds. In order to count the valence electrons it is necessary just to subtract the number of core electrons from the total number of electrons.
For the first three rows in the periodic table, the number of core electrons is simply equal to the number of elements in the rows above the row in which an element is found. For example, since there are no rows above hydrogen or helium these elements have no core electrons. Hydrogen (H) has one valence electron and helium (He) has two. Lithium (Li), element number 3, has two core electrons so lithium has 1 valence electron. Beryllium (Be) also has two core electrons and so Be has two valence electrons. The entire second row of the periodic table has two core electrons so the number of valence electrons follows a simple pattern: the first element in the row has one, the second has two, and so on until the end of the row where the last element has eight valence electrons.
The number of valence electrons for the third row can be figured out in exactly the same way only the number of core electrons is now 10 (2 electrons for the first row and 8 for the second). In the fourth row things become more complicated. By thinking only about groups 1 and 2 and groups 13 through 18, though, we can use a pattern to help us decide on the number of valence electrons. That is, within each group every element has the same number of valence electrons as the element above it in that group.
 For example, lithium (Li) has one valence electron so therefore sodium (Na), potassium (K), rubidium, (Rb) and cesium (Cs) all have one valence electron, too. This pattern does not work for the elements scandium (Sc) through zinc (Zn) but it does work for all of the elements in groups 13 through 18. For example, because we know that fluorine (F) has seven valence electrons we also know that iodine (I) has seven valence electrons.
For example, lithium (Li) has one valence electron so therefore sodium (Na), potassium (K), rubidium, (Rb) and cesium (Cs) all have one valence electron, too. This pattern does not work for the elements scandium (Sc) through zinc (Zn) but it does work for all of the elements in groups 13 through 18. For example, because we know that fluorine (F) has seven valence electrons we also know that iodine (I) has seven valence electrons.
Once you know how to count valence electrons you are ready to draw Lewis dot diagrams. Simply write the symbol for the element and surround it with a number of dots equal to the number of valence electrons. The Lewis dot diagram of chlorine is shown at right. Notice how the dots are spaced out so they are easy to count.
Answer the following questions using one or more complete sentences. Everyone in the group must write down complete answers. Discuss among your group members what the best way to answer the question is and then write it down.

Lewis dot diagrams are a simplified way to show how the electrons are arranged in their outer shell. This is where chemical reactions take place. Dots are placed around the element symbol in the following way: only the electrons in the valence shell are shown, they are added in pairs up to a total of four pairs. Here is an example:
Draw the Lewis Dot Structures for neutral atoms of elements 1-18:| H |
Lewis Dot Diagrams |
He | |||||
| Li | Be | B | C | N | O | F | Ne |
| Na | Mg | Al | Si | P | S | Cl | Ar |
| a. H and O | c. H and S |
| b. H and N | d. C and Cl |
Write names for the following molecular compounds.
Write the formulas for the following molecular compounnds: