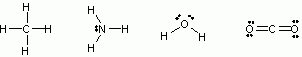Here is another way to approach things.
- The following is a methodical way to draw Lewis structures of molecules whose atoms follow the octet rule.
- It’s nice because it tips you off to exceptions to the octet rule like the expanded octet.
- Step 1: Count the electron pairs needed
* Hydrogen wants 1 pair
* Group II’s want 2 pairs
* GroupIII’s want 3 pairs
* Everyone else wants 4 pairs (unless you have an expanded octet) - Step 2: Find the total number of covalent bonds
possible
* Total # of possible covalent bonds = sum of the valence e– divided by 2
* Remember to add or subtract electrons according to any ionic charge - Step 3: Calculate the actual number of covalent
bonds
* Actual C.B.s = step 1 - step 2
If you don’t get enough bonds to draw the molecule then it’s an expanded octet. - Step 4: Calculate the number of σ bonds, π
bonds, and non-bonding pairs
* # of σ bonds = # of atoms in the molecule - 1
* # of π bonds = Actual C.B.s (step 3) - # of σ bonds
* # of non-bonding pairs = possible C.B.s (step 2) - Actual C.B.s (step 3) - Step 5: Draw the molecule (Here are some typical examples)
CH4 NH3 H2O CO2
step 1: 8 7 6 12
step 2: 4 4 4 8
step 3: 4 3 2 4
step 4:
σ 4 3 2 2
π 0 0 0 2
nbp 0 1 2 4
step 5: