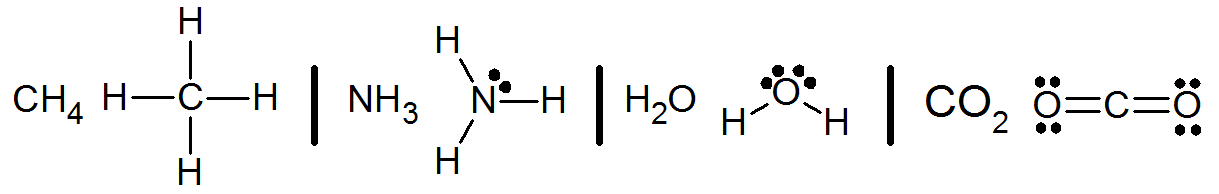
|
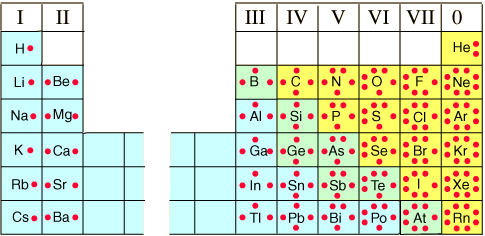
The number of valence electrons an atom has is determined by the arrangement of the electrons shells and the total number of electrons found in a neutral atom. The complete description of how this is determined is unecessary when the aim is to be able to draw molecular structures. A simple way to count valence electrons is to look at the group number. In the figure at right the old group numbers are given as roman numerals. The number of dots arranged around each atomic symbol gives the number of valence electrons. Note that the value of the group number and the number of valence electrons is equal. Modern periodic tables have changed group number labels so that groups III – VIII (zero in this figure) are now labeled as groups 13 – 18. Still, the pattern—once seen—is easy to remember.
Atoms form bonds with one another based on the number of valence electrons they have. The general rule is known as the octet rule. This rule governs the number of electrons an atom needs to borrow from other atoms in order to have a full complement of eight electrons in its outermost shell. The reason for this rule is that eight electrons is a sort of magic number. When an atom has eight valence electrons it is in a lower energy state than when it does not. An atom in a Lewis structure has an octet when the total shared and un-shared electrons is eight.
Conventionally, electrons are considered in pairs (an exception is a molecular radical with an odd number of electrons). Electron pairs in a bond are represented as a line between the atomic symbols: H—O—H. Non-bonding pairs (also called lone pairs) are represented by a pair of dots near the atomic symbol they belong to (for example, :N—F3). Lone pair (also called non-bonding pair) electrons are valence shell electrons that do not participate in bonding. There are two main things that need to be true about a correct Lewis structure for a molecule:
- All valence electrons for all atoms, and accounting for an ionic charge, must be included in the final drawing. A correct molecular drawing must have no more and no fewer electrons than the total valence electrons calculated at the start of the drawing process.
- All atoms, except hydrogen, must have an octet in the final correct drawing. An octet means that the atom has 8 electrons that are either non-bonding pairs or electrons shared with other atoms (both electrons in a bond count toward the total of eight). Hydrogen atoms only make one bond and never have lone pairs of electrons. There are some exceptions to this rule. See instructions below.
How to draw Lewis structural drawings of molecules:
- Count the number of valence electrons for each of the atoms in the molecule and add them together. The number of valence electrons an atom has depends on the group number. Group 1 elements (H, Li, Na, etc.) have 1 electron. See the image above for other groups.
- Electrons have a negative charge. When they are added to a molecule they give the molecule a negative charge. For each negative charge add one electron to the total of valence electrons. For each positive charge, subtract one electron for the total of valence electrons. The electrons involved in giving an ion a charge do not change anything about how electrons should be arranged in the molecular drawing.
- Organize the atoms around a central atom in a hub and spoke arrangement. Hydrogen is never the central atom. Atoms other than the central atom are referred to as ligands.
- Draw a single line between each ligand atom and the central atom to represent bonds between those atoms. Each bond requires one electron pair (two electrons).
- Add lone pairs of electrons to the ligand atoms until each one has three lone pairs, making a total of eight. Add remaining electrons as lone pairs on the central atom. Remember you must have exactly the number of valence electrons you calculated in steps 1 and 2. Count to make sure they are all present in the molecule. At this point you have a first draft of your molecule drawing.
- Sometimes the central atom will not have an octet. If it is a metal atom from groups 1, 2, or 13 then this is normal. Group 1 atoms only make one bond, group 2 atoms make two bonds, and group 13 atoms make three bonds. For other non-metal atoms they must have an octet. To give the central atom an octet more electrons will need to be shared. Erase or cross out lone pairs on a ligand atom and make an additional bond to the central atom. This makes a double bond. If the central atom still does not have an octet then you may make a double bond with another ligand atom or a triple bond with the same one, as appropriate. Ligand halogens (Group 17, F, Cl, Br, and I) do not make double or triple bonds.
- Double-check: do all atoms have an octet?
- If not, rearrange your electrons to make it so. If necessary, re-draw the skeleton of your molecule to make it possible to give every atom an octet.
- Central atoms may sometimes have 10 or even 12 electrons. Since such molecules exist we must allow some exceptions to the octet rule. Atoms for which this is possible are P, S, Cl, As, Se, Br, Te,, I, Kr, and Xe. The elements C, N, O, and F always have an octet in every structure.
- Always prefer the octet rule, if it is possible to follow it. Only exceed an octet if there is no other option.
- To account for the charges of molecular ions and to understand the distribution of electrons within molecules we use the concept of formal charge. The formal charge of an atom in a molecular structure drawing is calculated based on a comparison of the original number of valence electrons for that atom to the number of electrons assigned to it alone in the structure. Calculate it as
follows:
- Count one half of the total number of bonding electrons attached to the atom.
- Count the total number of lone pair electrons.
- Add up the previous two quantities and subtract from the number of valence electrons for that element based on its group number. This is the formal charge.
Use the Usual Bonding Patterns table to help you decide whether your structures look normal. Some molecules will have atoms that break these patterns but most will not. Think of drawing structures as solving a puzzle and the usual bonding patterns are the puzzle pieces. Molecular ions will always have one or more atoms that break the usual bonding patterns, though it is usually not necessary to have more than an octet on the central atom.
An easy way to find formal charge is to draw a circle around an atom in a Lewis Structure and count the electrons inside the circle (counting only half of each bond as being inside the circle). Subtract the result from the number of valence electrons to find formal charge. Note formal charges next to atoms by using a small + or – number in a circle.
Most atoms in most structures will have a formal charge of zero. Molecular ions, or polyatomic ions as they are also called, must have formal charges. The sum of all formal charges in a molecule must equal the charge of the molecule. For neutral molecules this means any formal charges other than zero must add up to zero. For ions it means they must add up to the overal charge.
Formal charge is simply a way to decide whether electrons are equally or unequally shared in a molecule. When formal charge is zero it means that the number of electrons shared or owned by an atom equals the number of valence electrons. When an atom has a formal charge that is negative it means that the atom has taken electrons away from its neighbors and is sharing unfairly by taking more electrons. Usually atoms with high electronegativity will do this. In other words, non-metals and especially non-metals at the furthest right-hand extreme of the periodic table. When an atom has a formal charge that is positive it means that the atom has given away electrons to its neighbors and is sharing more generously. Usually atoms with low electronegativity will do this. In other words, metals may be found to occur in some molecules with a positive formal charge.
Not all molecular model drawings (Lewis diagrams) require the calculation of formal charge. You only need to calculate formal charge under any one of the following conditions:
- Calculate formal charge on all atoms in a molecule that is a polyatomic ion. Remember, formal charges must all add up to the overall ionic charge.
- Calculate formal charge also when at atom does not follow its usual bonding pattern. In the Usual Bonding Patterns table you will find the symbols for atoms from Period 2 along with the number of bonds they are usually found to make and the number of non-bonding pairs (lone pairs) of electrons they usually have. Use this as a guide when drawing molecules. When a molecule seems to require a deviation from the usual bonding pattern you should calculate formal charge for that atom.
| Usual Bonding Patterns | |||||
| Group Number |
No. of Valence Electrons |
Example Atoms |
No. of Bonds |
Number of Lone Pairs |
Examples |
| 1 | 1 | H Li Na K | 1 | 0 |
|
| 2 | 2 | Be Mg Ca | 2 | 0 |
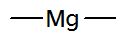
|
| 13 | 3 | B Al | 3 | 0 |
|
| 14 | 4 | C Si | 4 | 0 |
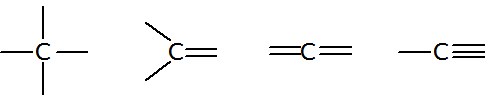
|
| 15 | 5 | N P As | 3 | 1 |
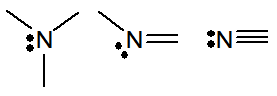
|
| 16 | 6 | O S Se | 2 | 2 |
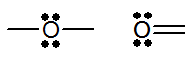
|
| 17 | 7 | F Cl Br I | 1 | 3 |
|