Counting and Ratios
Atoms are the smallest pieces of chemical substances that there are. They come in different varieties called elements and although they can change their arrangements in uncountable ways, atoms themselves can be counted. In fact, counting atoms is central to the understanding of chemistry.
One way that counting is important in chemistry is that atoms can be counted in the ways they build up molecules. For example, molecules of oxygen, the gas that keeps us all alive, are made of exactly two atoms. That’s why the chemical formula for oxygen is O2. Water molecules have exactly two atoms of hydrogen and one atom of oxygen: H2O.
Atoms, or ions, are also counted in ionic compounds. The formula for such compounds does not give an exact number of atoms in each piece of the compound. Instead, there are at least two pieces to ionic compounds: a positive ion called a cation and a negative ion called an anion. Sodium chloride, common table salt, has the formula NaCl, which indicates that the ratio of sodium ions (Na+) to chloride ions (Cl–) is one to one. Another ionic compound, copper (II) nitrate (Cu(NO3)2) has two nitrate ions (NO3–), which have four atoms each, for every one copper (II) ion (Cu2+). When ionic compounds dissolve in water and when they take part in chemical reactions they do so as separate ions so it’s important to bear in mind the number of ions that compose a compound.
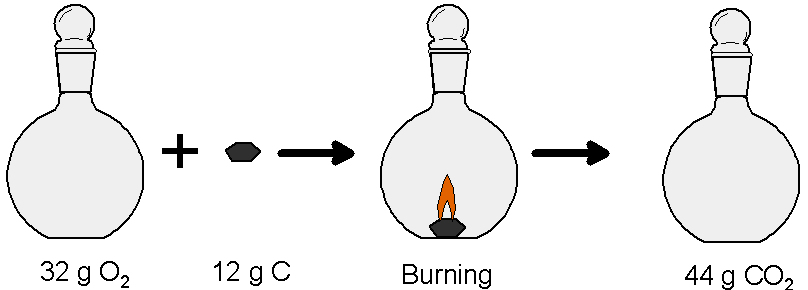
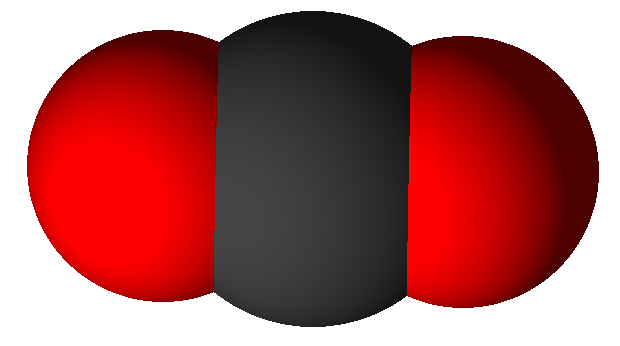
Another way that counting atoms is important is in chemical reactions. Balanced chemical equations describe chemical reactions by showing how atoms are rearranged in a chemical change while preserving the number of each kind of atom in the reaction. Reactants have bonds that are broken as they are transformed into the products, in which new bonds have formed. Two elements can often combine in different ways, with different numbers of atoms for each element. For example, carbon dioxide (CO2) is made when equal numbers of carbon atoms (C) and oxygen molecules (O2) combine. We say that they combine in a one to one ratio as seen in this chemical equation:
|
|
Carbon dioxide (CO2) |
|
|
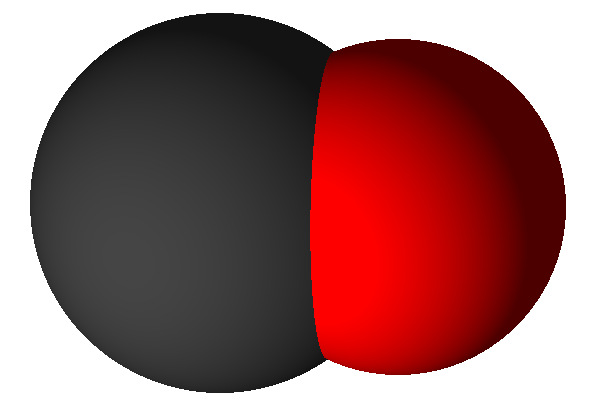
Carbon monoxide (CO) |
In this equation oxygen reacts with carbon in a ratio of 1 oxygen molecule to 1 carbon atom.
Carbon can also combine to form carbon monoxide, an altogether different molecule. Carbon monoxide (CO) molecules have one atom of each kind. When carbon reacts with oxygen to make carbon monoxide it does so with half as much oxygen per carbon atom as when they combine to make carbon dioxide. That is, in a 1 oxygen molecule to 2 carbon atoms ratio, as seen in this chemical equation:
These different ratios are rather abstract, but they are at the heart of our understanding of the fundamental laws of chemistry. Ratios by numbers of atoms and molecules are important in a practical sense, too. Carbon dioxide is a waste product of the way our bodies turn food into heat and the energy we need to think and to move. Our bodies also use it to maintain the correct pH in our blood, so it is an important molecule. Of course, if we breathe too much of it, carbon dioxide can harm us. Still, it’s a relatively harmless molecule with its ratio of one carbon atom for every two oxygen atoms. Carbon monoxide, on the other hand, with its one to one ratio, is a deadly poison. Even at very low concentrations it can cause death from a lack of oxygen. The carbon monoxide molecule binds to the oxygen-carrying proteins in red blood cells and keeps them from being able to transport oxygen.
Counting and Mass
The numbers of atoms per molecule and the ratios in which atoms combine in chemical reactions are clear and simple whole numbers. This is because atoms are objects that can be counted in whole numbers: there is no such thing as half of an atom. The problem is that atoms are so small and so numerous that we can never actually count them in the same way that you can count bananas or doughnuts. To see why, consider how many molecules of water (H2O) there are in a tablespoon (about 15 mL). There are about
This is far too many to count. Even counting 100 at a time, that is at 100 per second, it would take one and a half trillion centuries to count all of them! But if they are so numerous, and they can’t be counted, then where did this fantastic number come from? It came from the fact that it is possible to count things by weighing them. Consider the problem of ensuring that every ream of paper produced in a factory must have 500 sheets. One way the paper could be counted would be by weight. Given the average weight of a single sheet, 500 sheets would have a weight 500 times as big and so, without touching and counting each sheet, it is possible to know that there are 500 sheets.
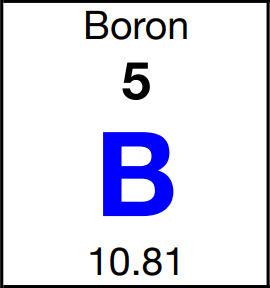
Atoms are counted by mass and it is interesting to see how this was set up. The starting point is the average atomic mass that is included in every box for every element on the periodic table. Boron, for example, has an average atomic mass of 10.81 amu. Atomic mass units (amu) are tiny units of mass used only for atoms and subatomic particles. They can be used to count small numbers of atoms. Ten atoms of boron would have a mass of 108.1 amu. One hundred atoms would have a mass of 1,081 amu. These are still very small numbers of atoms and masses too small to measure on a lab balance. By experiments that determine the number of atoms and molecules through chemcial reactions and the properties of gases, scientists are able to count atoms and to relate the number of atoms to the mass. And so it is possible to determine the conversion factor between atomic mass units and grams:
6.02 × 1023 amu = 1 gram
The number 6.02 × 1023 is called Avogardo’s Number. In the same way that 12 is the number of items in a dozen, Avogadro’s Number is the number of items in a chemical unit for counting items known as the mole. A mole is a group of items numbering 6.02 × 1023. The word mole is a word used to represent a number, just like the words dozen, million, billion, or trillion. One mole of atomic mass units is equal to one gram.
1 mole of amu = 1 gram
This number is key to counting atoms in a practical way for laboratory work. Here is how it works. Find the mass of a mole of atoms in atomic mass units:
10.81 amu 6.02 × 1023 ——————————— × —————————————— = 6.5076 × 1025 amu/mol 1 B atom 1 mol
This number of amu is very large so in order to have a reasonable mass for a mole of boron atoms we will convert from amu to grams. A mole of boron atoms, that is, 6.02 × 1023 of them, will have a mass we can work with:
6.5076 × 1025 amu 1 g ————————————————— × —————————————— = 10.81 g/mol 1 mol B atoms 6.02 × 1023 amu
This mass is a mass in grams for a large number of boron atoms, but instead of counting them individually, we count the atoms in groups. We count them in moles. The number 10.81 g/mol is the molar mass of the element boron. Molar mass is the mass in grams of one mole of a chemical substance, that is, it is the mass in grams for 6.02 × 1023 molecules. The units are g/mol and so the molar mass is a conversion factor that allows chemists to calculate the number of atoms in moles given the mass of a sample in grams or to calculate the mass of a sample in grams given the number of items in moles.
Formula Weight and Molar Mass
In order to convert the mass of a chemical into the number of moles, which is a way of counting the number of items, it is necessary to calculate the formula weight. The formula weight is the sum of all of the masses of the atoms in a chemical formula. Here are two examples:
| H2O | (2 × H) + (1 × O) | (2 × 1.008) + (1 × 15.999) = 18.02 |
| Fe(NO3)3 | (1 × Fe) + (3 × N) + (9 × O) | (1 × 55.845) + (3 × 14.007) + (9 × 15.999) = 241.857 |
The final number for each chemical is the molar mass, which is a conversion factor for that chemical to convert between a mass and the number of items in moles. It is an unchanging characteristic for a chemical substance, just like the density, color, and melting point. The molar mass is used to find out how many items you have when you weigh a sample of a material or to calculate the mass for the number of moles you need for a specific experiment.
Molar mass is a conversion factor that turns a mass in grams into a number of moles or vice versa. This is valuable in the lab because the number of moles can’t be measured directly. Instead, chemists measure masses. So to find out how many moles of a chemical there are in a given mass, you use the molar mass to convert from mass to moles, as in the following example:
Example 1
You have a beaker that weighs 42.71 g. You add some water and the mass increases to 63.28 g. How many moles of water are in the beaker?
First, find the mass of water:
63.28 g – 42.71 g = 20.57 g of water
Second, calculate the molar mass of water:
(2 × 1.008) + (1 × 15.999) = 18.02 g/mol
Third, convert grams to moles using the molar mass:
1 mol H2O
20.57 g H2O × ---------- = 1.14 mol H2O
18.02 g
On the other hand, at times a specific number of moles will be needed for an experiment or to mix a solution with a specific concentration. In that case, you convert the moles of the chemical that you need into a mass in grams, which it is easy to measure out in the lab.
Example 2
You need 2.5 mol of water for an experiment. To measure this you need to know how many grams of water this is.
First, calculate the molar mass of water (see ex. 1)
Second, use the molar mass to convert from mol to g
18.02 g
2.5 mol H2O × ---------- = 45.05 g H2O
1 mol H2O
Counting Atoms within Molecules
The chemical formulas of molecules show the number of each type of atom found in the molecule. This number can of course be scaled up. For example, ten water molecules have a total of 20 hydrogen atoms, and 10 oxygen atoms. Similarly, 10 mol H2O have a total of 20 mol H atoms and 10 mol O atoms. You can do this calculation as a sort of unit conversion by taking the chemical formula to count numbers of atoms within molecules according to their chemical formula. For example:
Example 3
2 mol H 1 mol O 1 mol O ------------- and ------------- and ----------- 1 mol H2O 1 mol H2O 2 mol H
At times it is useful to be able to count atoms within samples of substances in the lab, where we rely on mass to measure things. Molar mass enables you to calculate the number of moles of hydrogen atoms there are in 75 g of water, for example:
Example 4
How many moles of hydrogen atoms are there in 75 g of water?
First, calculate the molar mass of water (see ex. 1)
Second, use the molar mass to convert from g to mol
1 mol H2O
75 g H2O × ---------- = 4.16 mol H2O
18.02 g
Third, calculate the number of hydrogen atoms in moles
2 mol H
4.16 mol H2O × ----------- = 8.32 mol H atoms
1 mol H2O
Another way to use this idea is to calculate the mass of a single element that is present in the mass of a substance. Silver nitrate (AgNO3) is a common laboratory chemical also used in the development of black and white photographic films and papers. If you wanted to calculate the mass of silver atoms in a supply of this chemical then you can use this idea of counting moles of atoms within molecules, as in this example:
Example 5
How many grams of silver (Ag) are there is 5.0 g of silver nitrate (AgNO3)?
First, calculate the molar mass of silver nitrate:
(1 × 107.87) + (1 × 14.007) + (3 × 15.999) = 169.874 g/mol
Second, calculate the number of moles of silver atoms:
1 mol AgNO3 1 mol Ag
5.0 g AgNO3 × ------------- × ------------- = 0.0294 mol Ag atoms
169.874 g 1 mol AgNO3
Third, calculate the mass of silver atoms:
107.87 g
0.0294 mol Ag x ------------- = 3.18 g Ag
1 mol Ag
Using Avogadro’s Number
Generally, chemists do not use Avogardo’s Number (6.02 × 1023) very much. The whole point of it is to change numbers representing atomic mass units per atom to grams per mole so that atoms and molecules can be counted by weight. However, it is helpful in developing your understanding of the size of atoms and molecules. It is such a large number that it shows just how small atoms and molecules are in comparison to the objects we are used to in our lives. To help with this comparison, it is useful to imagine having a mole of ordinary objects like eggs, pennies, or grapefruits. If it were possible to have a mole of chicken eggs they would cover the entire surface area of the Earth…four miles deep. If you were rich enough to own a mole of pennies then they could be stacked in groups 400 pennies high in a disk that reaches from the surface of the Earth all the way to the orbit of the moon. (The bank teller would hate you for bringing them in for a deposit; it is enough money so that if distributed evenly every person on Earth would have over 1 trillion dollars.) Finally, how many grapefruits would it take to fill the entire volume of the Earth? The volume of the Earth is about 1021 m3. It takes about 500 grapefruit to fill up a box one meter on a side (1 m3). A mole of grapefruit would be large enough to fill the entire volume of the planet Earth. There is an excellent working out of the consequences of having a mole (the number) of moles (the small burrowing mammal) and you should read it: https://what-if.xkcd.com/4/.
As you can see, having a mole of anything that has a normal size is nearly inconceivable. There is another reason to use Avogadro’s Number, and that is anytime you need to know something like how many atoms are equivalent to the thickness of a hair or a piece of aluminum foil. Granted, these occasions are rare, but they do come up from time to time. Here is how to handle it:
Example 6
A calculation has determined that a piece of aluminum foil has a thickness made up by 1.08 × 10–19 mol of aluminum atoms. How many individual atoms is this?
6.02 × 1023
1.08 × 10–19 mol × -------------- = 6.5 × 104 or 65,000 aluminum atoms
1 mol
Example 7
In order to determine the number of atoms that cross the thickness of a piece of copper wire you might first calculate the number of copper atoms in a piece of wire. Find the number of copper atoms in 0.25 g of copper (Cu).
First, find the number of moles of copper atoms:
1 mol Cu
0.25 g Cu × ---------- = 0.00381 mol Cu
63.546 g
Second, calculate the number of atoms using Avogadro's Number:
6.02 × 1023
0.00381 mol Cu × ------------- = 2.30 × 1021 Cu atoms
1 mol
Example 8
How many grams of uranium (U) are there if you have 1.2 × 1023 atoms?
First, find the number of moles of uranium atoms:
1 mol
1.2 × 1023 U atoms × ------------ = 0.20 mol U
6.02 × 1023
Second, calculate the mass using the molar mass of uranium:
238.03 g
0.20 mol U × ---------- = 47.6 g U
1 mol
Comparing Numbers of Moles
It is important to remember that for the purpose of studying chemistry the important quantity is the number of moles, not the mass. It is the number of molecules reacting with each other that predicts the outcome, not the mass. For that reason it is valuable to have an understanding of relative numbers of particles. This can be counterintuitive, as the following example shows:
Example 9
Which sample has a larger number of particles? In other words, which one has a larger number of moles, 8.06 g of hydrogen (H2) or 79.85 g of iron(III) oxide (Fe2O3)?
1 mol
8.06 g H2 × ---------- = 4.00 mol H2
2.01559 g
1 mol
79.85 g Fe2O3 × ---------- = 0.5000 mol Fe2O3
159.69 g
There are 8 times more particles of H2 in 8.06 g than there are particles of Fe2O3 in 79.85 g. This may seem odd, that there are so many more molecules of hydrogen than units of iron(III) oxide. But it makes sense because hydrogen has a molar mass that is much smaller. Think of it this way, if you have 2 kg of coconuts than you have fewer nuts than if you have 2 kg of walnuts. And this is just because walnuts are so much smaller than coconuts.
Questions and Problems
Comprehension Questions
Answer the following questions using one or more complete sentences. Show work for any calculations.
- How is the counting of atoms important in understanding the composition of chemical compounds?
- Why is counting atoms and molecules important for understanding chemical reactions using chemical equations?
- Show how the mass in atomic mass units of a mole of carbon atoms can be converted into grams using the conversion factor given in the text.
- How is a mole similar to a dozen?
- How does the molar mass of a chemical relate mass to the number of molecules?
- If you know how many moles of a chemical you have, and you know its chemical formula, how do you calculate the number of moles of each different type of atom in the chemical? For example, to the find the number of moles of chlorine atoms in a certain number of moles of iron(III) chloride (FeCl3).
- Why is it that there are more moles of aluminum atoms in 10 g of aluminum (Al) than moles of iron atoms in 10 g of iron (Fe)?
Moles: Numbers and Masses
Molar Masses
For each of the following elements or compounds calculate the mass of 1 mole of particles of that substance. Express answers in units of g/mol.For example: the molar mass of HCO3– is H: 1 × 1.008 g/mol + C: 1 × 12.01 g/mol + O: 3 × 16.00 g/mol = 60.02 g/mol
See the Formula Weight section in the text.
- Al
- O2
- NaCl
- H2O
- CaCO3
- AlPO4
- Mg(OH)2
- Fe2(CO3)3
Putting Molar Masses to Work
The previous exercise requires you to find the mass in grams of one mole of a chemical substance. This mass has a special name in chemistry: the molar mass. When you work with this number, as with any number in science, you need a unit. The unit of molar mass is grams per mole (g/mol). To have a mole of sand (SiO2) you measure out approximately 60 g. But chemists seldom work with exactly one mole.
How many moles of SiO2 are there in 12 g? How many grams do you weigh out if you need 2.4 moles of SiO2? Just use dimensional analysis to figure it out:
60 g 1 mol
2.4 mol × -------- = 144 g 12 g × -------- = 0.20 mol
1 mol 60 g
In the following problems, find the number of moles given the number of grams. Find the number of grams given the number of moles. See examples 1 and 2.
- 47 g of O2
- 112 g of NaCl
- 91 g of H2
- 14 mol of H2O
- 0.35 mol of Fe2O3
- 42 mol of Mo(PO4)2
Comparing Numbers of Particles
Write an equal sign (=), a greater than sign (>), or a less-than sign (<) between each of the following pairs. Determine which sign is appropriate by comparing the number of moles of each member of the pair. That is, compare the number of atoms or molecules in each member of the pair. For example, there are more atoms in 28 g of N2 than in 2 g of C. This is because 1 mole of N2 has a mass of 28 g. One mole of C has a mass of 12 g. Since there are only 2 g of C and this is equal to 0.17 mol there are more molecules of N2 in 28 g of N2 than in 2 g of C.
See example 9.
- 13.5 g Al 2 g He
- 10 g Fe 10 g Br
- 64 g NaCl 32 g H2O
- 14 g Fe2O3 14 g U
- 207 g Au 207 g Pb
- 1 g O 2 1 g N2
- 10 g H 2 10 g PbCl2
- 12 g H2O2 11 g CH4
Counting Atoms
Answer the following questions by performing a calculation. See examples 3, 4, and 5.
- How many moles of Cl atoms are in 1 mol of MgCl2?
- How many moles of N atoms are there in 0.75 mol of Al(NO3)3?
- How many moles of C3H8 contain 3.5 mol of hydrogen atoms?
- How many moles of Fe2O3 contain 0.27 mol of oxygen atoms?
- How many moles of potassium atoms are in 58 g K2S?
- How many moles of oxygen atoms are in 100 g O2F2?
- How many moles of oxygen atoms are in 15 g H2SO4?
- How many moles of H2O contains 42.0 g of hydrogen atoms?
Big Numbers
Answer the following questions by performing a calculation. You will need to use Avogardro’s Number (6.02 × 1023 per mole) for every question. See examples 6, 7, and 8.
- How many individual units of sodium chloride are in 2 mol NaCl?
- How many individual units of iron(III) oxide are in 1.5 mol Fe2O3?
- How many moles are there if you have 2.3 × 1023 atoms of lead (Pb)?
- How many moles of water are there if you have 4.2 × 1022 molecules?
- How many individual units of sodium chloride are in 42 g NaCl?
- How many individual units of iron(III) oxide are in 84.0 g Fe2O3?
- How many grams of C3H8 is equal to 3.1 × 1023 molecules?
- How many grams of H2O is equal to 5.4 × 1025 molecules?