You have learned about electron structure, ions, the octet rule, chemical stability, and chemical reactivity. All of this has been from the point of view of individual atoms.

|
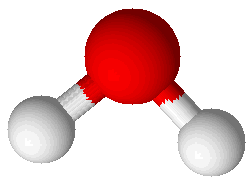
| H2O |

|
| NH3 |

|
| CH3CH2OH |
Now you will turn to learning about combinations of atoms. Atoms can combine in a couple of different ways. One way that atoms combine is as molecules. Molecules are covalently bonded collections of atoms that behave as a unit. Some common molecules are water (H2O: 2 H atoms and 1 O atom), carbon dioxide (CO2: 1 C atom and 2 O atoms), oxygen (O2: 2 O atoms), ozone (O3: 3 O atoms), nitrogen (N2: 2 N atoms), hydrogen peroxide, (H2O2: 2 H atoms and 2 O atoms), alcohol (CH3CH2OH: 2 C atoms, 6 H atoms and 1 O atom), and ammonia (NH3: 1 N atom and 3 H atoms).
Notice that some of these molecules contain only one kind of atom. These molecules are not compounds but elements. There are seven elements whose most common form is a diatomic molecule: H2, N2, O2, F2, Cl2, Br2, and I2. The elemental form of oxygen can be either O3 or O2. We breathe O2 but O3 is poisonous (but essential for absorbing some of the sun’s UV radiation). When elements can occur in more than one form then those forms are called allotropes. Another common example of an allotrope is diamond: pure carbon can have the form of charcoal or graphite but it can also be a diamond. It is simply a matter of how the atoms are arranged.
Most molecules are compounds: substances in which atoms of different elements are chemically bonded to one another. Compounds are formed through the chemical reactions of elements and other compounds with each other. Molecular compounds are characterized by consisting of collections of atoms which are all covalently bonded to one another. Covalent bonds form when atoms share their electrons more-or-less equally. The mutual attraction of two atomic nuclei for the shared electrons creates the bond. They are not the same as ionic compounds. In ionic compounds the connection between atoms comes about because of the attraction of positive and negative charges for each other. In ionic bonds whole atoms have negative and positive charges due to the loss or gain of electrons. These opposite charges are attracted to each other, creating the bond.
Molecular compounds have certain defining characteristics. The atoms
in a molecule are bound together and move and act as a unit. They
have relatively low melting points (H2O mp = 0°C), low boiling
points (H2O bp =
100°C) and make poor conductors of 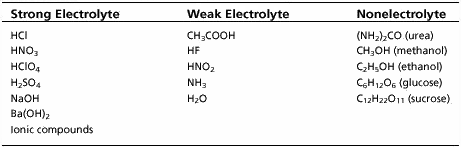 electricity even when melted into a
liquid. In addition, molecular compounds are
non-electrolytes.
electricity even when melted into a
liquid. In addition, molecular compounds are
non-electrolytes.
An electrolyte is a substance that, when dissolved in water, allows the solution to conduct electricity. They do this because electrolytes are ionic compounds (made of positive and negative ions) and these ions break up when in water. Once the ions can move they can carry an electric current.You may be familiar with electrolytes from ads for sports drinks that claim to replace electrolytes lost during exercise. The electrolytes they are talking about are usually Na+, K+, Mg2+, and Ca2+. The sodium they get from table salt: NaCl. A non-electrolyte may dissolve in water but the resulting solution does not conduct electricity because molecular compounds cannot break up into ions.
When ions form through the process of a chemical reaction they can combine to make ionic compounds. Ionic compounds have a different kind of bond than molecular compounds. When sodium metal reacts with
chlorine gas (2Na + Cl2 —> 2NaCl) the neutrally charged sodium atoms each lose one electron and the neutrally charged chlorine atoms each gain one electron. It is an even exchange and both kinds of atom become charged. Sodium atoms have a +1 charge and chlorine atoms have a –1 charge.
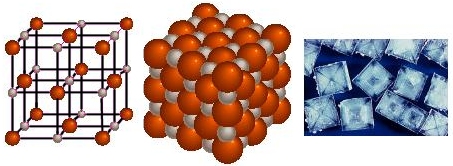
You may remember that objects with opposite electric charges attract one another. Sodium ions and chloride ions attract each other very strongly and form ionic bonds. The ions Na+ and Cl- have a one-to-one ratio in NaCl but you can never have just one unit of NaCl.
In the pictures above are three representations of NaCl. In the first you can see that for each of the small atoms (Na+) there are six of the large atoms (Cl-). The same is true for each of the Cl- ions. All of the ions are really in contact with each other, like in the middle picture. Salt is always a crystal with all of the positive charges surrounded by negative charges and all of the negative charges surrouned by positive charges.
Ionic compounds have relatively high melting points (NaCl mp = 801°C), high boiling points (NaCl bp = 1413°C) and make poor conductors of electricity. That is, when they are solids. When ionic compounds melt they make excellent conductors of electricity because the ions are free to move around. Moving ions can carry electric current in the same way that electrons in a wire carry electric current. The cations and anions can move freely and are attracted to electrical wires carrying a current. Their motion toward the wire they are attracted to serves to carry electrical charge from one wire to another, making it possible for electric current to flow.
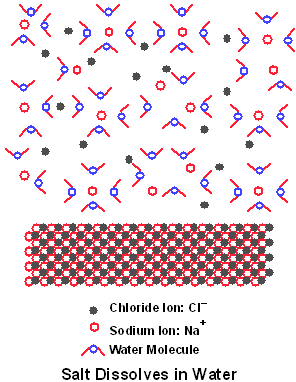
Ionic compounds are strong electrolytes. Strong electrolytes work well to conduct electricity when they dissolve because they break up completely into cations and anions. The ions in liquid solution can move around, just as they could if the ionic compound is melted. Weak electrolytes, which form ions from molecular compounds, only partially break up into ions. The majority of a sample of a weak electrolyte stays whole and is neutral. Only a small fraction of the total sample breaks up into ions that can carry a current. An example of what happens when a strong electrolyte dissolves is shown at right. When sodium chloride (NaCl) dissolves it turns complete into Na+ and Cl- ions: NaCl --> Na+ + Cl-.
Formulas for ionic compounds are always written in lowest terms. In NaCl there is one sodium cation for every chloride anion. You could write Na2Cl2 or Na42Cl42 and the ratio of Na+ to Cl- would be the same. But the point is to make things as simple as possible so you will just write NaCl every time you mean sodium chloride.
The reason there is always just one Na+ for every Cl- is that each ion has one unit of
charge (+ or -). In ionic compounds the charges always balance. That
is, all ionic compounds are electrically neutral. For
Na+ and Cl- there is a +1 charge and a -1
charge. These add up to zero, making the combination electrically
neutral. Take another example: calcium fluoride. Calcium has a +2
charge (Ca2+) and fluoride
has a -1 charge (F-)—you could predict this also
from the electron 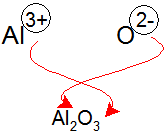 configurations. To write the formula you would need to have
two F- for every
Ca2+ because (2 × -1)
+ (+2) = 0. This makes the compound electrically neutral and you
write the formula as CaF2.
An easy way to do this is to realize that the subscript on the
cation will be equal to the charge on the anion and that the
subsript on the anion will be equal to the charge on the cation. Use
aluminum oxide as an example: Al3+ and O2- combine to form Al2O3.
configurations. To write the formula you would need to have
two F- for every
Ca2+ because (2 × -1)
+ (+2) = 0. This makes the compound electrically neutral and you
write the formula as CaF2.
An easy way to do this is to realize that the subscript on the
cation will be equal to the charge on the anion and that the
subsript on the anion will be equal to the charge on the cation. Use
aluminum oxide as an example: Al3+ and O2- combine to form Al2O3.
Take a good look at the ions and their names in the table you received with this packet. Notice that nearly all of the cations are metals and that most of the anions are nonmetals. Ionic compounds tend to form between metals and nonmetals. Some of the anions and one of the cations contain more than one atom. These ions are polyatomic ions. The ions that consist of only one atom are monatomic ions. It will be helpful to you to become familiar with the more commonly used names since naming compounds correctly is necessary for good communication. The names of ionic compounds are always named with the cation first, followed by the anion. It is left to you to figure out the formula because once you know the name of an ion you can figure out its charge. If you know the charge of both ions in a compound then you can figure out its formula.
Molecular compounds are named slightly differently. They are formed mostly from nonmetallic elements and come in discrete molecular units. When a compound contains more than one of a particular atom then Greek numerical prefixes are used in the name to make that clear.
Carbon, sulfur and nitrogen all form several different compounds with oxygen. It is important to specify which one you mean. It is common to add the letters -ide to the end of the name of a molecular compound: HCl is hydrogen chloride, SiC is silicon carbide. Some compounds do not use the prefix system: CH4 is methane, H2O is water, NH3 is ammonia. What these compounds have in common is that they contain hydrogen. They are very common compounds and just go by their non-sytemmatic names.
Some elements, especially metals, can have more than one charge. Iron commonly has a +2 or a +3 charge. These ions are called iron(II) (Fe2+, say “iron two”) and iron(III) (Fe3+, say “iron three”). Other metal ions that take different charges at different times can be named in the same way.
The covalent bonds and ionic bonds that you have read about so far are only the two extreme ends of a spectrum of types of chemical bonds. Linus Pauling (1901 - 1994), one of the most influential chemists of the 20th century, studied the chemical bond in great detail. In his studies of the chemical bond he found it useful to describe bonds according to where electrons in bonds spend most of their time. In some bonds, electrons are exchanged entirely: ionic bonds. In others, electrons are shared completely equally: covalent bonds. In completely equal covalent bonds the atoms share electrons just the way people share a cake: two equal slices. This type of bond is called non-polar covalent. There are also bonds that are somewhere on a spectrum in between. These bonds are called polar covalent bonds. Nearly all bonds between atoms are at least slightly polar. In polar covalent bonds the electrons spend more time closer to one atom than to the other. Polarity in a molecule is when part of the molecule has a slight negative charge and another part has a slight positive charge.
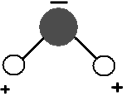
The degree of polarity of a chemical bond can be calculated using the relative scale of electronegativity developed by Pauling. Electronegativity is the ability of an atom to attract toward itself the electrons in a bond. Atoms that are more electronegative tend to draw the negatively charged electrons toward themselves. Water is a great example of a compound with very polar bonds. Oxygen is more electronegative than hydrogen and therefore draws electrons toward itself. This causes there to be a partial positive charge on hydrogen and a partial negative charge on oxygen (see the picture). These charges are fractional, not full electric charges. Water (H2O) is an excellent example of a polar molecule.
| Electronegativity and Bond Character | ||
| >2.0 | —> | ionic bond |
| 0.5 - 2.0 | —> | polar covalent bond |
| ≤0.4 | —> | non-polar covalent bond |
To find out how polar a bond is use the electronegativity values from the chart on the next page. If the difference between two values is greater than 2.0 then the bond is ionic. If the difference is between 0.5 and 2.0 then the bond is polar covalent. If the difference is between 0 and 0.4 then the bond is non-polar covalent.
Electronegativity ties everything together that you have read so far. Bonds between atoms of very different electronegativities are ionic. Bonds between atoms of similar electronegativities are non-polar covalent. In between there are the polar covalent bonds.
|
Electro- negativity |
Name | Symbol |
Atomic Number |
| 2.1 | Hydrogen | H | 1 |
| 0 | Helium | He | 2 |
| 1.0 | Lithium | Li | 3 |
| 1.5 | Beryllium | Be | 4 |
| 2.0 | Boron | B | 5 |
| 2.5 | Carbon | C | 6 |
| 3.0 | Nitrogen | N | 7 |
| 3.5 | Oxygen | O | 8 |
| 4.0 | Fluorine | F | 9 |
| 0 | Neon | Ne | 10 |
| 0.93 | Sodium | Na | 11 |
| 1.2 | Magnesium | Mg | 12 |
| 1.5 | Aluminum | Al | 13 |
| 1.8 | Silicon | Si | 14 |
| 2.1 | Phosphorus | P | 15 |
| 2.5 | Sulfur | S | 16 |
| 3.0 | Chlorine | Cl | 17 |
| 0 | Argon | Ar | 18 |
| 0.82 | Potassium | K | 19 |
| 1.0 | Calcium | Ca | 20 |
| 1.3 | Scandium | Sc | 21 |
| 1.5 | Titanium | Ti | 22 |
| 1.6 | Vanadium | V | 23 |
| 1.7 | Chromium | Cr | 24 |
| 1.5 | Manganese | Mn | 25 |
| 1.8 | Iron | Fe | 26 |
| 1.9 | Cobalt | Co | 27 |
| 1.9 | Nickel | Ni | 28 |
| 1.9 | Copper | Cu | 29 |
| 1.6 | Zinc | Zn | 30 |
| 1.6 | Gallium | Ga | 31 |
| 1.8 | Germanium | Ge | 32 |
| 2.0 | Arsenic | As | 33 |
| 2.4 | Selenium | Se | 34 |
| 2.8 | Bromine | Br | 35 |
| 0 | Krypton | Kr | 36 |
| 0.82 | Rubidium | Rb | 37 |
| 1.0 | Strontium | Sr | 38 |
| 1.2 | Yttrium | Y | 39 |
| 1.4 | Zirconium | Zr | 40 |
| 1.6 | Niobium | Nb | 41 |
|
Electro- negativity |
Name | Symbol |
Atomic Number |
| 1.8 | Molybdenum | Mo | 42 |
| 1.9 | Technetium | Tc | 43 |
| 2.2 | Ruthenium | Ru | 44 |
| 2.2 | Rhodium | Rh | 45 |
| 2.2 | Palladium | Pd | 46 |
| 1.9 | Silver | Ag | 47 |
| 1.7 | Cadmium | Cd | 48 |
| 1.7 | Indium | In | 49 |
| 1.8 | Tin | Sn | 50 |
| 1.9 | Antimony | Sb | 51 |
| 2.1 | Tellurium | Te | 52 |
| 2.5 | Iodine | I | 53 |
| 0 | Xenon | Xe | 54 |
| 0.79 | Cæsium | Cs | 55 |
| 0.89 | Barium | Ba | 56 |
| 1.10 | Lanthanum | La | 57 |
| 1.12 | Cerium | Ce | 58 |
| 1.13 | Præsodymium | Pr | 59 |
| 1.14 | Neodymium | Nd | 60 |
| 1.17 | Promethium | Pm | 61 |
| 1.20 | Samarium | Sm | 62 |
| 1.21 | Europium | Eu | 63 |
| 1.22 | Gadolinium | Gd | 64 |
| 1.23 | Terbium | Tb | 65 |
| 1.23 | Dysprosium | Dy | 66 |
| 1.23 | Holmium | Ho | 67 |
| 1.24 | Erbium | Er | 68 |
| 1.25 | Thulium | Tm | 69 |
| 1.27 | Ytterbium | Yb | 70 |
| 1.27 | Lutetium | Lu | 71 |
| 1.3 | Hafnium | Hf | 72 |
| 1.5 | Tantalum | Ta | 73 |
| 1.7 | Tungsten | W | 74 |
| 1.9 | Rhenium | Re | 75 |
| 2.2 | Osmium | Os | 76 |
| 2.2 | Iridium | Ir | 77 |
| 2.2 | Platinum | Pt | 78 |
| 2.4 | Gold | Au | 79 |
| 1.9 | Mercury | Hg | 80 |
| 1.8 | Thallium | Tl | 81 |
| 1.9 | Lead | Pb | 82 |
Answer the following questions using one or more complete sentences. Everyone in the group must write down complete answers. Discuss among your group members what the best way to answer the question is and then write it down. All members must write down the answer or risk losing participation points.
The periodic table tells you the atomic number, the average atomic mass and also how many valence electrons an element has. It can also reveal certain other trends in the properties of the elements. By making a graph you will explore the trend of electronegativity values in the periodic table.

When you dissolve salt in water you make what chemists call a solution. Salt dissolves in water because water consists of molecules with very polar bonds. Salt has ionic bonds. Because both substances are highly polar, they mix easily together. Oil consists of molecules with very non-polar bonds (C-C and C-H). Because of this, it will not mix with water. That is, it will not dissolve in water. Chemists refer to this phenomenon by saying that “like dissolves like”. Based on the polarity of the bonds, which compounds out of those below do you think will dissolve in water? In oil?
Binary Ionic Compounds - Type I
Binary ionic compounds contain two ions, a cation and an anion,
that react to form a compound. The steps for naming these
compounds are:
Additional information: the names of ionic compounds do not use prefixes to show how many of each ion there are in each formula unit of the compound. If you are asked write a formula when you are given the name of a compound then you must know the charge of the ions involved in order to make the charges balance.
Binary Covalent Compounds - Type II
Binary covalent compounds are those that do not involve metals or
ions. You can recognize these compounds because they usually
contain only non-metals. Here are the steps for naming a binary
covalent compound:
Additional information: The first word of the formula never uses mono, so CO is Carbon Monoxide and is not Monocarbon Monoxide.
Some names just need to be memorized:
Do not draw lines! Just write the letter of the name next to the correct formula.
In the following exercises you will be given chemical formulas. Name the compound. For example, Na2CO3 is Sodium Carbonate and NaHCO3 is Sodium Hydrogen Carbonate.
Build a correctly written chemical formula from the two ions given. Write the name of the compound, too. For example, Ca2+ and F- => CaF2 Calcium Fluoride and NH4+ and SO42- => (NH4)2SO4 Ammonium Sulfate.
In the following exercises you will be given names of compounds. Write the formula. For example, Potassium Bromide is KBr and iron(III) oxide is Fe2O3