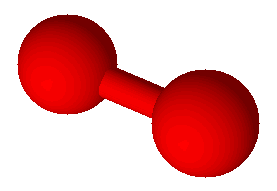
Oxygen (O2)
Hydrogen
Chloride (HCl)
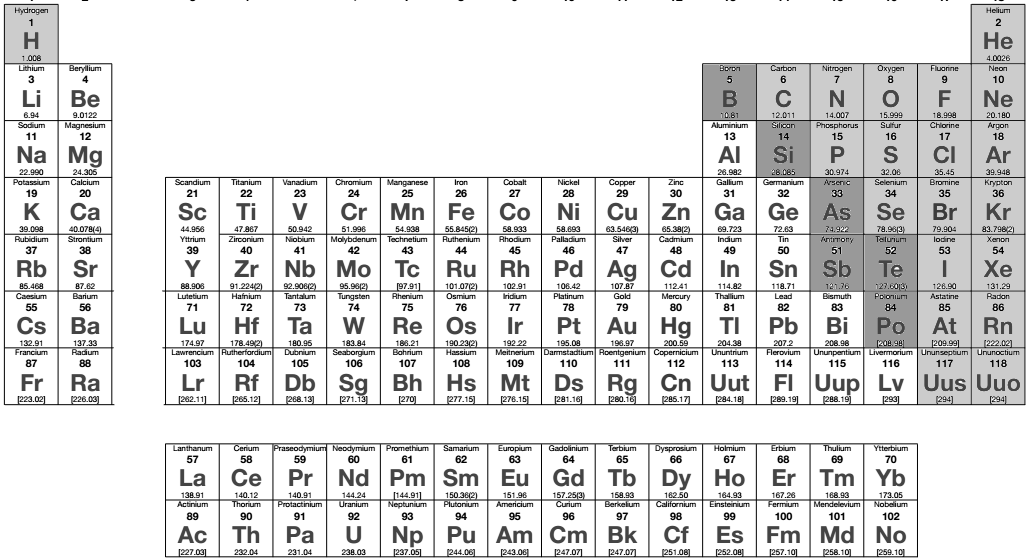
The periodic table of elements shows basic information about all the different types of atoms that make up all of ordinary matter. There is a basic division in the table between elements considered to be metals and elements considered to be non-metals. Metals make up most of the elements in the periodic table. In the graphic at right the metals are white. The non-metals are light-grey. A few elements fall in between these categories and are called semi-metals. These are colored dark gray. Familiar metals include iron, silver, gold, copper, and zinc. Usually gray or silver in color, metals are good conductors of heat and electricity and most are not brittle. Metals deform instead of breaking when struck hard with a hammer. Only one metal, mercury (Hg), is a liquid at room temperature. Familiar non-metals include carbon (charcoal), sulfur, oxygen, and neon. Non-metals that are solids tend to be brittle and are usually poor conductors of heat and electricity. Many pure non-metals are gases and one, bromine (Br2), is a liquid. For a material to be called an element it must be made of only one kind of atom.

Oxygen (O2) |

Hydrogen Chloride (HCl) |
Atoms can bond together in a number of different ways. As elements, non-metal atoms can bond together to form molecules, which are collections of atoms that move as a group. Metallic elements bond together with a special type of bond called metallic bonds. These bonds form vast networks that allow electrons to move from atom to atom like boats in a vast sea dotted with islands. Compounds are formed when atoms of different elements bond to one another. When non-metals bond to other non-metals they do so by sharing electrons. The electrons in the bond spend most of their time between the nuclei. Being negatively charged, electrons attract both atomic nuclei, which are positively charged. Because the electrons that create the bond come from the valence shell of the atoms this type of bond is called a covalent bond.
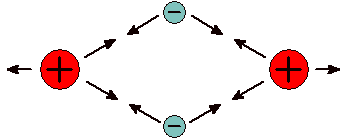
Covalent Bond Large atomic nuclei bond due to attractions to tiny electrons. |
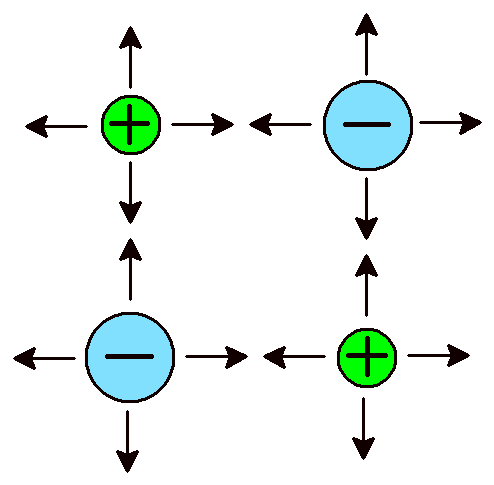
Ionic Bonds A network of attractions between ions. |
Covalent bonds hold atoms together because shared electrons attract both atoms. When atoms are held together by covalent bonds they form molecules. Oxygen is an example of an element which naturally forms molecules; in this case with two atoms each: O2. Oxygen is a diatomic molecule, one with just two atoms. There are seven elements, including oxygen, that exist naturally in their pure form as diatomic molecules. Commit these to memory: hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2). There are compounds with diatomic molecules, too, such as HCl. Hydrogen chloride has one atom of hydrogen and one atom of chlorine and they are bound together by a covalent bond.
Molecules of compounds are collections of atoms covalently bound together and which move as a unit. Molecules themselves can bond to one another by intermolecular bonds. This type of bonding is what is responsible for the ability of molecular compounds to become liquids or solids. The molecules stick to one another in solids and liquids. When they evaporate the molecules remain whole but the bonds between them are broken. Intermolecular bonding will not be a focus of the current activity.
Another type of bond is at the opposite end of the chemical bond spectrum, the ionic bond. Ionic bonds form between metal atoms and non-metal atoms and come about because positively and negatively charged atoms attract due to their opposite charges. The atoms themselves have either gained or lost one or more electrons. When atoms lose electrons they become positively charged. Metals characteristically form positively charged ions. Chemists refer to positive ions by the name cation. When atoms gain electrons they become negatively charged. Non-metals form negative ions, also called anions. Ionic bonds refer to strong attractions between ions of opposite charge. Ionic bonds do not allow atoms to form molecules. Instead, the atoms create a network of alternating cations and anions. Sodium chloride (NaCl) is an excellent example of a familiar ionic compound. Its atomic structure consists of repeating sodium (Na+) and chloride (Cl–) ions. A single crystal of granulated salt might contain 1 × 1018 cations and 1 × 1018 anions (that’s a 1 followed by 18 zeroes). Regardless of the total number, there is always one cation for every anion.
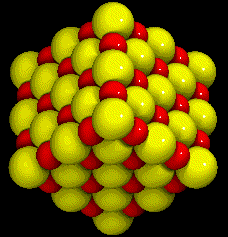
|
| NaCl Crystal |
Ionic compounds are combinations of atoms of different elements that are bound together by ionic bonds. They are always a combination of at least one metal with at least one non-metal. The formulas of ionic compounds do not show the number of atoms of each element found in a molecule of the compound. Ionic compounds do not form molecules. Instead, the formula of an ionic compound shows the lowest whole-number ratio of ions in the compound. In sodium chloride (NaCl) there is one sodium ion for every chloride ion. In calcium chloride (CaCl2) there is one calcium ion for every two chloride ions. Ionic compounds are characterized by having very high melting points and boiling points. Sodium chloride, for example, melts at 801°C and boils at 1413°C. Ionic compounds are strong electrolytes, poor conductors of electricity as solids, and excellent conductors as liquids. Crystals of ionic compounds vary in the details of their structure but mainly they consist of alternating positive and negative ions, as in the image of NaCl at right. The sodium atoms in the crystal are smaller and darker.
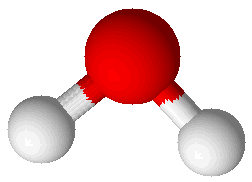
Water (H2O) |
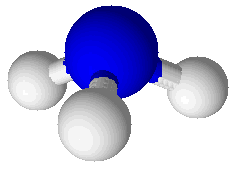
Ammonia (NH3) |
Molecular compounds are combinations of atoms bound together by covalent bonds to make molecules. Molecules are groups of atoms bound together in a particular pattern with a particular number of each kind of atom. For example, molecules of water (H2O) have a central oxygen atom bound to two hydrogen atoms. A picture showing a water molecule is displayed at right. Bonds in this picture are shown as cylinders that connect the spheres representing atoms. The oxygen atom is larger than the two hydrogen atoms. Another molecular compound is ammonia (NH3), which has one nitrogen atom and three hydrogen atoms. It is shown at right, below the water molecule. Molecular compounds are characterized by having relatively low melting points and boiling points. Ammonia melts at –78°C and boils at –33°C. Ammonia is a gas in its pure state at room temperature. Water melts at 0°C and boils at 100°C: this common, familiar compound is actually very unusual in that its melting and boiling points are so high for such a small molecule. Molecular compounds are either non-electrolytes or weak electrolytes. They are poor conductors of heat and most do not conduct electricity. When molecular compounds solidify as crystals they do so as a result of intermolecular bonds. This type of attraction between molecules is much weaker than either covalent bonds or ionic bonds. One way to compare the strength of intermolecular bonds to covalent bonds is to compare the energy required to boil water to the energy required to break water into hydrogen and oxygen gases. To form H2 and O2 by breaking the covalent bonds between oxygen and hydrogen atoms in water molecules requires 7 times as much energy (286 kJ/mol) as to break all the bonds holding liquid water molecules together (40.7 kJ/mol).
Binary molecular compounds are compounds made of two elements. Some examples are water (H2O), ammonia (NH3), carbon dioxide (CO2), and methane (CH4). Of these four examples, only one is usually know by its formal, systematic name. The International Union of Pure and Applied Chemistry (IUPAC) is the organization which publishes the conventions used to name chemical compounds. For this reason chemists all over the world use the same names for the same compounds. Having a system to determine the single, unique name for a compound is very important because without such a system confusion could result. If different chemicals had the same or nearly the same name it would lead to mistakes in which the wrong chemical is added to a reaction.
Naming binary molecular compounds is quite simple. More complicated compounds are much more difficult to name but will not be covered in this activity. The name of a compound gives both the names of the elements that compose it and the number of atoms of each element found in the compound. This is accomplished by using prefixes that indicate the number. The prefixes are shown in a box above.
The first step in naming a compound given its formula is to recognize whether or not it is molecular or ionic. Molecular compounds are those made of non-metals. Compound formulas containing a metal atom are usually ionic and follow a different system for naming them. Once a compound is identified as molecular prefixes are assigned to each element’s name and the second element’s suffix is changed to –ide. The mono– prefix is never used for the first element in the name of a molecular compound. Some example formulas with names:
Notice in the names above that the final vowel of the prefix is dropped when used with oxygen. When the –ide suffix is added to an element’s name it sometimes shortens the name a bit. Sulfur becomes sulfide, phosphorus becomes phosphide, and nitrogen becomes nitride, and oxygen becomes oxide. Note that other elements’ names are not much changed: fluorine becomes fluoride, chlorine becomes chloride, etc. There are many molecular compounds that have common names. These compounds are rarely, if ever, referred to by their systematic names. Water (H2O), ammonia (NH3), and methane (CH4) are examples everyone should know. Be careful with compounds containing hydrogen. Some, like the ones just mentioned, have common names. Others have names that depend on whether or not the compound is dissolved in water. These compounds will be discussed later.
| Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 | |
| Li+ | Be2+ | B3+ | — | N3– | O2– | F– | |
| Na+ | Mg2+ | Al3+ | Si4+ | P3– | S2– | Cl– | |
| K+ | Ca2+ | Ga3+ | Ge4+ | As3– | Se2– | Br– | |
| Rb+ | Sr2+ | In3+ | Sn2+ / Sn4+ | Sb3– | Te2– | I– | |
| Cs+ | Ba2+ | Tl1+ / Tl3+ | Pb2+ / Pb4+ | Bi3+ / Bi5+ | — | — |
Some ions are single atoms, called monatomic ions. Non-metals are always negatively charged when they occur in compounds as monatomic ions. Metals are always positively charged in ionic compounds. Hydrogen is positively charged (H+) when it is part of an acid. When combined with a metal ion in an ionic compond hydrogen has a negative charge (H–) and is called the hydride ion. Many monatomic ions have a predictable and consistent charge in all compounds. The table at right shows the pattern in the periodic table for such ions:
Monatomic ions from the transition metals often have more than one possible charge in ionic compounds. For example, iron appears in some compounds with a +2 charge and in others with a +3 charge. Later this text will cover how to tell by looking at a formula whether it is one iron ion or the other. For now it is important to note two things: One, a number of metal elements from the middle of the periodic table have two or more possible ionic charges. Two, ions with different charges make compounds with different formulas and different properties. In the table below some selected elements are shown with the ionic charges they may have. Notice particularly that one of the ions of mercury (Hg) has two atoms: Hg22+. The most common ionic charge is bold and underlined.
| Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | Group 9 | Group 10 | Group 11 | Group 12 |
|
Sc
3+ |
Ti
4+ / 3+ |
V
5+ / 4+ / 3+ / 2+ |
Cr
6+ / 3+ / 2+ |
Mn
4+ / 2+ / 3+ |
Fe
2+ / 3+ |
Co
2+ / 3+ |
Ni
2+ / 3+ |
Cu
2+ / 1+ |
Zn
2+ |
|
Y
3+ |
Zr
4+ |
Ag
1+ |
Cd
2+ |
||||||
|
La
3+ |
Hf
4+ |
Au
3+ / 1+ |
Hg2+ / Hg22+ |
Some ions have more than one atom covalently bound together. These molecules with a
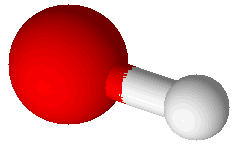
Hydroxide Ion (OH–) |
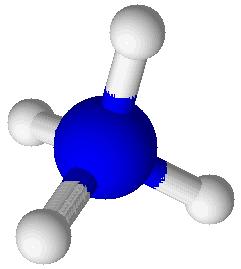
Ammonium Ion (NH4+) |
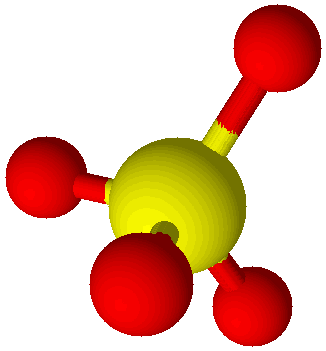
Sulfate Ion (SO42–) |
The important thing about building formulas of compounds out these ions is that they always occur as groups of atoms. For example, the compound sodium hydroxide (NaOH) has one sodium ion (Na+) and one hydroxide ion (OH–) but magnesium hydroxide (Mg(OH)2) has one magnesium ion (Mg2+) and two hydroxide ions. The formula ‘MgOH2’ is incorrect because it shows one magnesium ion, one oxygen atom and two hydrogen atoms. Since it is supposed to represent magnesium hydroxide it must show that there are two hydroxide ions. This is accomplished by using parentheses in the formula with a subscript outside to show how many of the polyatomic ion are in the formula. Parentheses are only used for polyatomic ions when there is more than one ion in the formula: notice that they are not used in the formula of sodium hydroxide.
Ionic compounds occur as a result of the balancing of charges between two ions. The compound as a whole must be electrically neutral with equal amounts of positive and negative charges. The formulas of ionic compounds always show the lowest whole-number ratio of cations and anions that create a neutral combination. Here are some visual examples to illustrate what this means:
| Cation | Anions | Cations | Anions | |||||||
| Ca2+ | Br– | Br– | Fe3+ | Fe3+ | SO42– | SO42– | SO42– | |||
| CaBr2 | Fe2(SO4)3 | |||||||||
Notice that charges are not written as part of the formula of each compound. Instead, the subscripts after each ion formula show how many ions are necessary to balance the charge. For calcium bromide (CaBr2) two bromide ions are needed for every calcium ion to make a neutral compound. For iron(III) sulfate (Fe2(SO4)3) two iron(III) ions are needed to balance three sulfate ions. Since three of these ions are needed in the formula the sulfate ion’s formula must be enclosed in parentheses with a three outside. That subscripted three shows that there are three whole sulfate ions in the formula of the compound.
To figure out a correct ion combination you must find the least common multiple (LCM) of the values of the charges on the ions. This is the most efficient way to get the correct total for the number of cations and the number of anions. Here are two examples: The formula of a compound made of copper(II) (Cu2+) ions and fluoride ions (F–) is determined by finding the LCM of 2 and 1, which is 2. This result means that the total amount of positive charge in the compound is +2 and the total amount of negative charge in the compound is –2. Only one copper(II) ion is needed to get +2 but two fluoride ions are needed to get –2. Therefore, the formula is CuF2. The combination of chromium(III) (Cr3+) and oxide (O2–) ions has an LCM of 6. To get +6 from a +3 ion requires two chromium(III) ions. To get a –6 from a –2 ion requires 3 oxide ions. Therefore the formula is Cr2O3.
Naming ionic compounds is actually fairly straightforward. To name a compound given its formula is simply to name the cation and then name the anion (leave off the word ‘ion’). Giving these names is enough to uniquely identify a formula because each ion always has the same charge. The charges must balance to make a neutral combination and there is always only one way for this to happen. The hard part of this is to be able to recognize ions in order to name them properly.
Positive ions are simply named for the element as follows. The element sodium (Na) makes only one possible ion, Na+. This ion is called the sodium ion. All other metals that only form one kind of ion are named in a similar way, for example, Ca2+ is called the calcium ion. Metals which have more than one possible charge are named by adding a roman numeral to indicate their charge. For example, Fe2+ is called iron(II) and Fe3+ is called iron(III). These are read aloud as ‘iron two’ and ‘iron three’.
In order to figure out which of the possible ions a metal in a compound might be it is necessary to look at the ion with which it is combined. Since anions always have only one possible ionic charge it is possible to deduce the charge on the metal ion based on how the ions are combined in the formula. For example, FeCl2 and FeCl3 are different compounds. The chloride ion always has a –1 charge so in the first of these two compounds that makes a total of –2. Therefore, the iron ion must have a +2 charge and is called iron(II). In the second compound there are three –1 ions so the iron ion is iron(III) since it must have a +3 charge.
There is an older system for naming ions of transition metals. The metal’s name is given in Latin and ions with the smaller of two possible charges use the suffix “–ous” and those with the larger charge use the suffix “–ic”. So the iron(II) ion is sometimes called the ferrous ion and the iron(III) ion is sometimes called the ferric ion. These suffixes are discouraged by the IUPAC but still appear in catalogs, chemical literature, and in lessons on nomenclature. See the ions reference sheet for further examples of these old-fashioned names.
There are only two polyatomic ions with a positive charge. One of these has already been mentioned: Hg22+. In its compounds this ion is reffered to as mercury(I). The second is the ammonium ion: NH4+. The names of these ions must be memorized.
| Groupu 13 | Group 14 | Group 15 | Group 16 | |
| Period 2 | Borate BO33– | Carbonate CO32– | Nitrate NO3– | n/a |
| Period 3 | n/a | Silicate SiO44– | Phosphate PO43– | Sulfate SO42– |
Monatomic anions are the simplest to name. The name of the element is used and the suffix is changed to –ide. So O2– is the oxide ion and I– is called the iodide ion.
Polyatomic ions fall into two categories. First, there are those that follow a regular pattern of naming. Second, there are those that simply must be memorized, such as cyanide (CN–), hydroxide (OH–), thiocyanate (SCN–), permanganate (MnO4–), chromate (CrO42–) and dichromate (Cr2O72–). Regular use and practice will take care of the second category. As for the first, all of them contain oxygen and are called oxyanions. Some elements combine with oxygen to make more than one oxyanion. Each one has its own unique name although oxyanions of the same element all have the same ionic charge. The oxyanion with the more oxygen atoms has the suffix –ate. There is a table of these oxyanions at right. If there is a second oxyanion of the same element with one fewer oxygen atom then it uses the suffix –ite. For example, nitrate is NO3– and nitrite is NO2–. Sulfate (SO42–) has the corresponding sulfite (SO32–) and phosphate (PO43–) has the corresponding phosphite (PO33–). None of the other –ate oxyanions in the table have a corresponding –ite oxyanion. The halogen elements, with the exception of fluorine, all make four different oxyanions. With the help of two prefixes each one can be given its own name. For the oxyanion with one fewer oxygen than the –ite anion the prefix hypo– is used. For the oxyanion with one additional oxygen atom compared to the –ate anion the prefix per– is used. The series of chlorine-based oxyanions is hypochlorite (ClO–), chlorite (ClO2–), chlorate (ClO3–), and perchlorate (ClO4–).
| Oxyanions of Chlorine, Bromine, and Iodine | |||
| hypochlorite ClO– |
chlorite ClO2– |
chlorate ClO3– |
perchlorate ClO4– |
| hypobromite BrO– |
bromite BrO2– |
bromate BrO3– |
perbromate BrO4– |
| hypoiodite IO– |
iodite IO2– |
iodate IO3– |
periodate IO4– |
When hydrogen (H+) combines with these oxyanions to form another polyatomic ion then it causes the negative charge to become more positive by one unit for each hydrogen added. It also changes the name. When one hydrogen is added, such as in hydrogen phosphate (HPO42–) the charge becomes –2 and the name gains the word ‘hydrogen’. Dihydrogen phosphate (H2PO4–) naturally has two hydrogen atoms added. If enough hydrogen atoms are added to make the polyatomic ion neutral then it has become an acid and must be named accordingly.
Some compounds produce hydrogen ions when they dissolve in water. Most of these compounds are known as acids and are given names to reflect this fact. Some acids are derived from covalent compounds and have a different name when dissolved in water than when they are pure substances. These are the binary acids, so-called because they contain only two elements in their formulas: hydrogen and one other. For example, hydrogen chloride (HCl) is a toxic gas with a biting odor that dissolves in water to make a strongly acidic solution. When dissolved in water this compound is known as hydrochloric acid. All such covalent compounds undergo a similar name change: the prefix hydro– is added to the name of the element bound to hydrogen, the suffix –ic is added to the end followed by the the word ‘acid’. So dihydrogen sulfide H2S (a toxic, horribly smelly gas) is known as hydrosulfuric acid when dissolved in water.
| Oxyanion | Acid | Name |
| ClO3– | HClO3 | Chloric Acid |
| ClO– | HClO | Hypochlorous Acid |
| CO32– | H2CO3 | Carbonic Acid |
| NO2– | HNO2 | Nitrous Acid |
| NO3– | HNO3 | Nitric Acid |
This last compound has a name easily confused with another common acid, sulfuric acid (H2SO4). Acids such as sulfuric acid are derived from oxyanions, such as those discussed above. Sulfuric acid is derived from the sulfate ion. Names for these acids follow a pattern. Acids formed by adding hydrogen ions to an oxyanion with an –ite ending change the ending to –ous and the word acid is added at the end. The oxyanion nitrite (NO2–) becomes nitrous acid (HNO2). Acids formed by adding hydrogen ions to an oxyanion with an –ate ending change the ending to –ic and the word acid is added at the end. So phosphate (PO43–) becomes phosphoric acid (H3PO4). Some other examples of oxyanions and their corresponding acids are given at right.
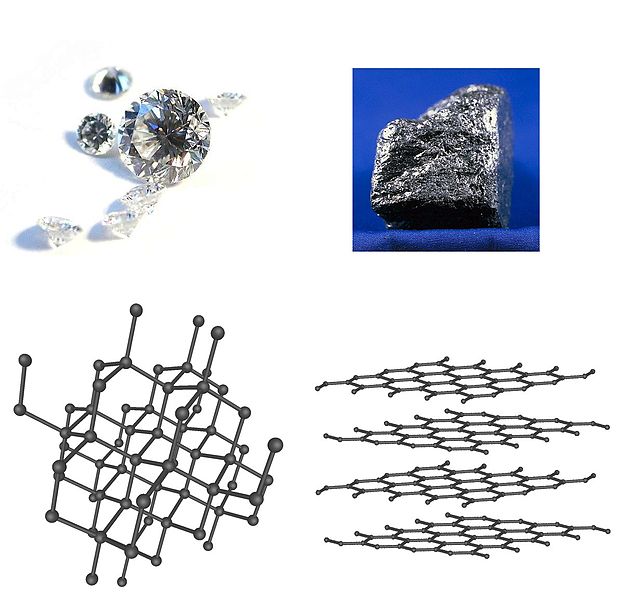
Diamond and Graphite image from Wikimedia Commons |
Finally, there is one more thing about chemical names that ought to be mentioned. Some elements occur in multiple forms on account of different ways in which their molecules are structured. These different chemical forms of the same element are called allotropes. Carbon has a wide variety of allotropes but two of the important ones are diamond and graphite. Pure diamonds are made of nothing but carbon atoms. The graphite in your pencil is actually a mixture of pure graphite and clay but the graphite itself is made of nothing but carbon atoms. The difference is in how the atoms are arranged. In diamonds the carbon atoms are all bound strongly to each other with four bonds per carbon atom. In graphite each carbon atom is bound to only three other atoms in two-dimensional sheets. Graphite can be rubbed off onto paper because these sheets are only loosely bound to one another.
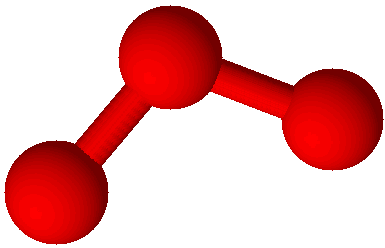
Ozone (O3) |
Oxygen has two common allotropes. One of them, O2, is illustrated on the first page of this document. The other, ozone (O3), is illustrated at left. Many living things cannot survive without oxygen, however ozone is a deadly poison. Ozone is not without its uses; in the Earth’s upper atmosphere a very small amount of ozone serves as a shield against some of the stronger ultraviolet light from the sun. Without it the surface of the land would be awash in dangerous radiation.
Earlier in this document molecular compounds were described as being either weak electrolytes or non-electrolytes. Ionic compounds were described as strong electrolytes. An electrolyte is a compound which, when dissolved in water, causes water to conduct electricity. Water itself, if very pure, is a poor conductor of electricity. But when an ionic compound dissolves in it the water becomes a much better conductor. The more of the ionic solid that dissolves, the better water can conduct electricity.
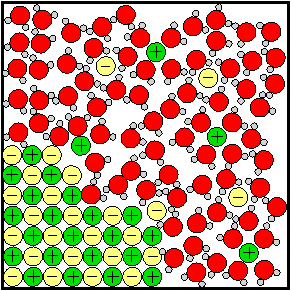
Salt Dissolving in Water |
The behavior of strong electrolytes is explained by the changes that occur at the molecular level when an ionic compound dissolves. Water molecules have positive and negative charges at opposite ends of each molecule. While they are small charges, smaller in size than the charge on a +1 or –1 ion, they are strong enough to be strongly attracted to ions. Because of this attraction they can pull the ions free from a crystal and surround them in the solution. Take a look at the illustration at right. There are also video clips avaiable at http://youtu.be/xdedxfhcpWo and http://youtu.be/dr4sFNzUVzI.
The water begins to be able to conduct electricity due to the now-mobile ions in the solution. The ions move around randomly when no electrical current is present. But when wires connected to a power source such as a battery are run into and out of the salty water current can flow because the ions organize their motion. Anions move toward the positively charged wire and cations move toward the negatively charged wire. The organized motion of ions in solution in the presense of an electrical current is what allows electricity to flow through salt-water. Weak electrolytes are molecular compounds that have the chemical property that they interact with water to increase the number of free ions in solution. They usually have a small part, such as hydrogen atom, which can become ionized. A group of weak electrolyte molecules remain almost 100% whole and only a tiny fraction of a percent dissociate into ions.
Answer the following questions using one or more complete sentences. Everyone in the group must write down complete answers. Discuss among your group members what the best way to answer the question is and then write it down.
Fill in the following table with neutral model formulas (like this: AB2) for each possible pair of ions. Use the least common multiple of the ionic charges to figure out the subscripts in the lowest whole-number ratio.
| B– | B2– | B3– | |
| A+ | |||
| A2+ | |||
| A3+ |
Write names for formulas and formulas for names of the following monatomic ions.
Write the names and formulas for all of the period four transition metal ions that you will find in a table in the text delivered with these questions. There are ten elements but many of them have more than one ion. Give the symbol, showing the charge, and the name of each one. For example, one chromium ion is Cr3+ and is called chromium(III).
Fill in the following table by creating ionic compound formulas for the ions given. Under each formula write the name of the compound. One has been done for you as an example. Remember: all ionic compounds have a combination of ions that is neutral.
| F– | Cl– | O2– | P3– | |
|
Na+
|
||||
|
K+
|
||||
|
Ba2+
|
||||
|
Fe3+
|
FeF3 iron(III) fluoride |
Fill in the following table by creating ionic compound formulas for the ions given. Under each formula write the name of the compound. One has been done for you as an example.
| OH– | CN– | CO32– | PO43– | |
|
NH4+
|
||||
|
Cu+
|
||||
|
Cu2+
|
||||
|
Ti4+
|
Ti(OH)4 titanium(IV) hydroxide |
Fill in the following table by creating ionic compound formulas for the ions given. Under each formula write the name of the compound. One has been done for you as an example.
| sulfide | sulfate | nitrate | nitrite | |
|
copper(I)
|
||||
|
copper(II)
|
||||
|
nickel(II)
|
||||
|
nickel(III)
|
Ni2S3 nickel(III) sulfide |
Write names for the following ionic compounds.
Write formulas for the following ionic compounds.
Write names for the following molecular compounds.
Write the formulas for the following molecular compounnds:
Fill in the following table with the formulas for the –ate oxyanions of the elements shown.
|
B:
|
C:
|
N:
|
—
|
—
|
|
— |
—
|
P:
|
S:
|
Cl:
(perchlorate) |